Translate this page into:
Ultrasound assessment of ovarian lesions: O-RADS approach
*Corresponding author: Radha Sarawagi, Department of Radiodiagnosis and Imaging, All India Institute of Medical Sciences (AIIMS), Bhopal, India. radha.radiodiagnosis@aiimsbhopal.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Yadav U, Sarawagi R, Patel A, Rahul S, Malik R. Ultrasound assessment of ovarian lesions: O-RADS Approach. Future Health. 2024;2:24–34. doi: 10.25259/FH_10_2024
Abstract
Lesions of ovarian or adnexal origin are common in all age groups, but there is significant variability in the interpretation of the words, definitions, and morphologic descriptions by the radiologists and the clinicians. There has also been a paradigm shift in the diagnosis and management of ovarian lesions during the last 20 years.
Ovarian-Adnexal Reporting and Data System (O-RADS) by the American College of Radiology (ACR) was introduced in 2018. ORADS US (ultrasonography) serves as a tool to simplify the characterization of adnexal lesions, minimize the use of misleading terminology, and aid in the management of such lesions. It maintains the six risk assessment categories (O-RADS US 0–5) with an increasing predicted risk of malignancy from O-RADS US 1 to 5.
In this pictorial essay, we briefly summarize the O-RADS and its descriptors, followed by representative ultrasound images to help clinicians understand what the O-RADS descriptors in the ultrasound report would mean.
Keywords
Ovary
Adnexa
Ovarian carcinoma
ORADS-2022 update
O-RADS
Ultrasound
INTRODUCTION
Ovarian cancer was estimated to be the third most common cancer among Indian women and eighth overall, as per the Globocan 2018 Fact sheet. It is also a leading cause of death from cancer in Indian women, with 3.34% (24,015) of all cancer deaths in India in the same year.1
There can be many types of adnexal lesions, ranging from benign to malignant tumors. Accurate diagnosis of adnexal masses is essential for clinical decision-making and avoiding unnecessary surgeries for low-risk lesions. Ultrasonography is widely used for predicting malignancy in adnexal lesions.
An ultrasound assessment aims to categorize these abnormalities into different groups based on their probability of being benign, indeterminate, or highly indicative of malignancy.
Ambiguous terms like complex cyst vary from hemorrhagic cyst to mucinous or serous cystadenocarcinoma. However, it does not specify the risk of malignancy. Therefore, the need for lexicon and descriptors and their risk of malignancy was assessed.
International Ovarian Tumor Analysis (IOTA) simple rules, IOTA Assessment of Different Neoplasia in the Adnexa (IOTA ADNEX), Gynecologic Imaging and Reporting and Data System (GI-RADS), and the Society of Radiology in Ultrasound consensus statement on adnexal lesions are just a few of the publications that offer an overview or comparison of the current adnexal lesion categorization systems [Figure 1].
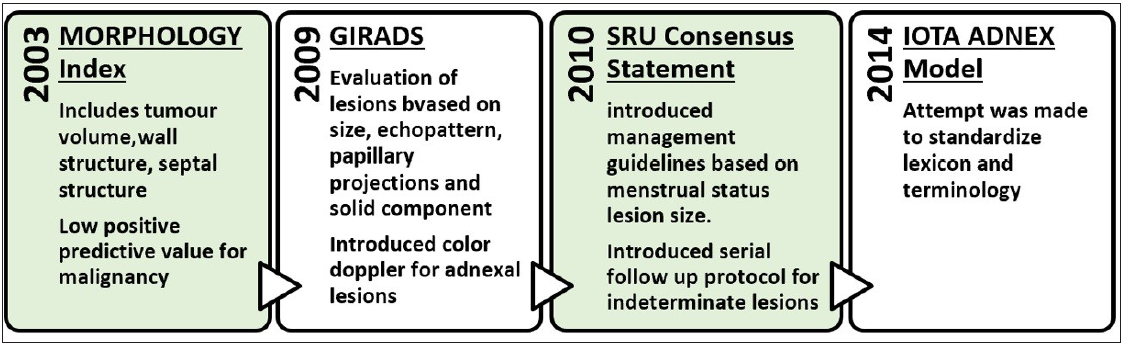
- Evolution of different categorization systems for adnexal lesions. GIRADS = Gynecologic Imaging and Reporting and Data System; SRU = Society of Radiology in Ultrasound; IOTA = International Ovarian Tumor Analysis
The stability of the cystic lesion in the follow-up scans over time, unfortunately, does not equate to benignity, which demonstrates a stepwise progression originating from a benign precursor to a borderline tumor. This requires evidence-based follow-up guidelines.2
Ovarian-Adnexal Reporting and Data System (O-RADS) by the American College of Radiology (ACR) was introduced in 2018. O-RADS is distinct from other ovarian lesion definition and management systems for the US because it has a dual modality-based lexicon and descriptors, that is, US and Magnetic Resonance Imaging (MRI), a shared language, and data-supported risk categorization. In a study evaluating the validity, reliability, and malignancy rates of O-RADS, GI-RADS, and IOTA simple rules, O-RADS demonstrated the highest sensitivity for malignancy when categories 4 and 5 were combined as predictors.3,4
Recent O-RADS US v2022 maintains the six risk assessment categories (O-RADS US 0–5) with an increasing predicted risk of malignancy from O-RADS US 1 to 5. [Tables 1 and 2]
| O-rads category | Risk of malignancy (in %) | Management |
|---|---|---|
| O-RADS 0 (Technically incomplete) | Not available | Repeat US or MRI |
| O-RADS 1 | 0 | None |
| O-RADS 2 | <1 | See Table 2 |
| O-RADS 3 (Low Risk) | 1-<10 |
If not surgically excised, consider follow-up US within six months. If solid, options include a US specialist (if available) or MRI (with O-RADS MRI score) |
| O-RADS 4 (Intermediate Risk) | 10-<50 |
Imaging Options include: • US specialist or • MRI O-RADS or • Per gyn-oncologist protocol Clinical- Gynaecologist with gyn-oncologist consultation or by gyn-oncologist solely. |
| O-RADS 5 (High Risk) | 350 | As per Gyn-oncologist, who may order an MRI or CT (eg., for staging) |
MRI = Magnetic resonance imaging; CT = Computed tomography; O-RADS = Ovarian-Adnexal Reporting and Data System; ACR-ORADS = American College of Radiology; US = Ultrasound
| ORADS score | Characteristics | Size | Premenopausal | Post-menopausal |
|---|---|---|---|---|
| 2 | Simple cyst |
> 3 cm to 5 cm > 5 cm to <10 cm |
- | Follow up in the US for 12 months. |
| Follow up US in 12 months. | ||||
| Unilocular cyst | <3 cm | None | Follow up US in 12 months | |
| Smooth margins and non-simple cysts (echoes/incomplete septations) | >3 -<10 cm | Follow up US in 6 months | ||
| Bilocular cyst | ||||
| Hemorrhagic cyst | 5-10 cm | Follow up US in 2-3 months | Confirm with follow-up US in 2-3 months or MRI O-RADS | |
| Dermoid | 3- 10 cm (if not excised ) | Irrespective of menopausal status – Follow up US in 12 months | ||
| Endometrioma | <10 cm (if not excised ) | Follow up for 12 months | Confirm with follow-up US in 2-3 months or MRI O-RADS, if still not excised, then follow up in 12 months. | |
| Para-ovarian/peritoneal inclusion cyst/hydrosalpinx | None | |||
O-RADS = Ovarian-Adnexal Reporting and Data System; MRI = Magnetic Resonance Imaging; US = Ultrasound
CLINICAL FEATURES AND ORADS APPLICATION
Depending on the size of the tumor and the stage of the disease, ovarian neoplasms can present with a wide range of nonspecific symptoms. Younger women are more likely to develop germ cell tumors than epithelial cell neoplasms, which typically appear in older women. The following are risk factors for epithelial ovarian cancer: advanced age, obesity, increased ovulatory cycles, hormone therapy, familial cancer syndromes (BRCA1, BRCA2, Lynch syndrome, Peutz-Jeghers syndrome), and fertility treatments.
Patients should be categorized as pre- or postmenopausal, as menopausal status is relevant for risk stratification or management.
• Postmenopausal defined as amenorrhea 3 1 year
• If uncertain or uterus is absent, manage as postmenopausal if age is > 50 years
When a potential adnexal lesion to be evaluated with US is shown by another modality (e.g., CT), O-RADS application to physiologic findings is advised for screening US examinations of patients who are at high risk (e.g., carriers of BRCA mutations).
O-RADS applies only to lesions involving the ovaries or fallopian tubes. If a pelvic lesion origin is indeterminate but suspected to be an ovarian or fallopian tube in origin, then the O-RADS system may apply. If a pelvic lesion is identified as not ovarian or tubal in origin, then the O-RADS system would be appropriate only in the case of a para-ovarian cyst or peritoneal inclusion cyst and, otherwise, does not apply.5
Pelvic inflammatory disease, ectopic pregnancy, and torsion of a normal ovary are to be excluded while using ORADS-US for categorization.6
ULTRASOUND TECHNIQUES
A transvaginal scan is recommended to optimally assess the ovarian and adnexal pathology. To assess possible pathologic problems outside the focal length of the vaginal transducer, the patients are examined with an abdominal transducer.
To prepare the transducer for a transvaginal scan, standard coupling gel is applied first. Next, a probe cover is inserted and again lubricated with coupling gel. When the uterus is retroverted, the transducer is inserted into the posterior vaginal fornix; when it is anteverted, it is inserted into the anterior vaginal fornix.
The observations include the size, form, and echotexture of the ovarian or adnexal masses in the transverse and sagittal planes. Despite these methods, the lesion is classified as ORADS 0 if the study is insufficient and cannot be assigned to an ORADS score or if additional imaging is needed.
NORMAL OVARY (O-RADS 1)
The O-RADS 1 category includes physiologic follicles [Figure 2a], and the corpus luteum of the ovary [Figure 2b] which may measure up to 3 cm and do not require further follow-up.
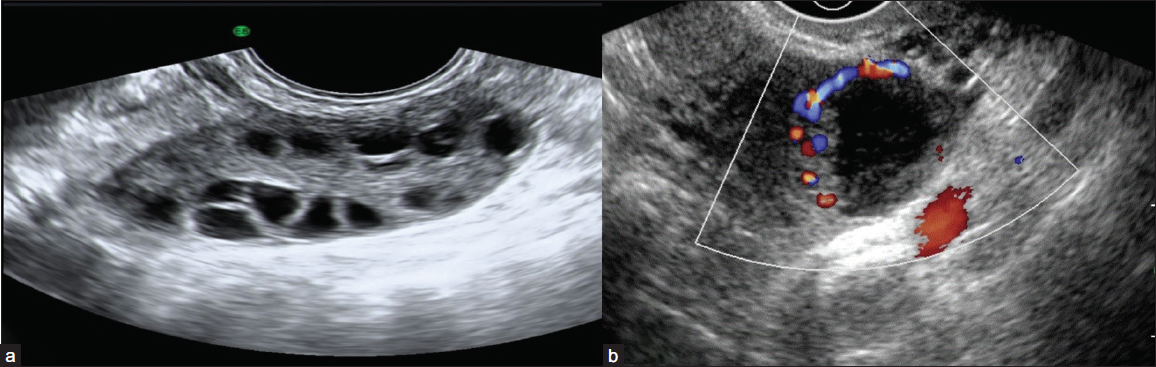
- (a) Grey-scale ultrasound image reveals a normal ovary with multiple peripheral follicles and homogeneous echotexture with a central echogenic medulla in a premenopausal female. (b) Color Doppler image shows a thick-walled cystic lesion with crenulated inner walls, few internal echoes, and peripheral vascularity in premenopausal females suggestive of corpus luteal cyst.
The normal postmenopausal ovary should not demonstrate these physiologic findings.
CLASSIC BENIGN LESIONS (O-RADS 2)
Classical lesions are described below [Figure 3]. The lesions that do not fall into this category should be described as explained by the lexicon below.6
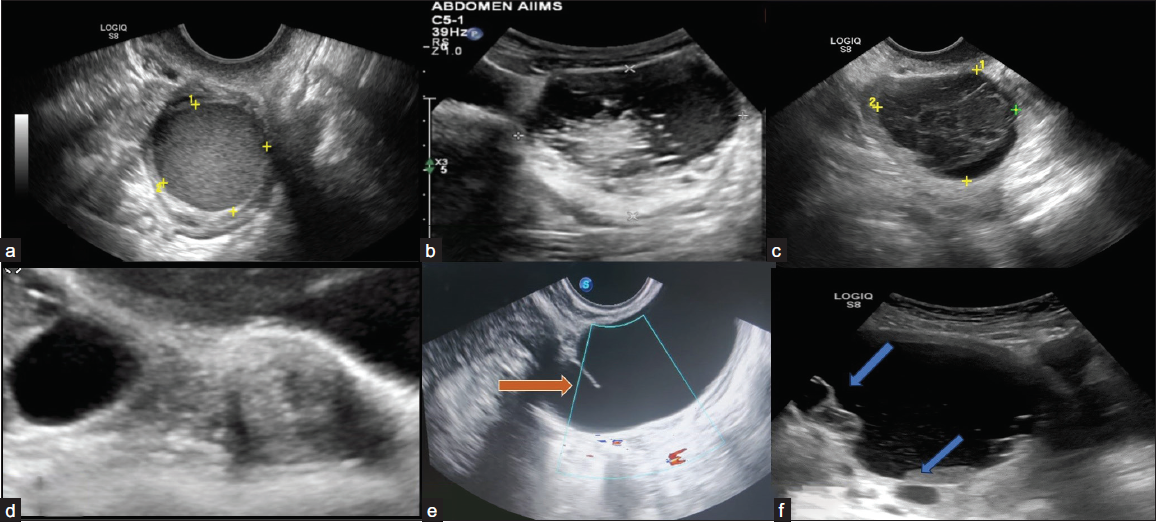
- Ultrasonography (USG) images of classically benign lesions. (a) Ultrasound image shows endometrioma characterized by a unilocular cyst within the ovary with homogeneous and evenly dispersed echoes throughout the entire cyst. (b) Grey-scale ultrasound images each show a typical dermoid cyst demonstrating a hyperechoic component with shadowing. Another typical feature of a dermoid cyst is hyperechoic lines and dots representing coiled hair. (c) Typical hemorrhagic cyst. A unilocular cyst with an internal reticular pattern of fine intersecting lines. (d) A simple cyst separates from the adjacent ovary, indicating para ovarian cyst. (e) Grey-scale longitudinal ultrasound image shows a tubular-shaped, fluid-filled structure with no internal echoes. An incomplete septation (arrow) representing a fold is seen, a feature of a typical hydrosalpinx. (f) Ultrasound image shows a peritoneal inclusion cyst characterized by unilocular fluid collection that conforms to adjacent pelvic organs with an ovary at the margin (upper arrow). There is a lack of a discrete limiting wall, no mural nodularity, and minimal internal debris (lower arrow).
Endometrioma
It is a well-defined cystic lesion with ground glass or homogeneous low-level echoes not taking any vascularity.
Dermoid
Cystic lesions with hyperechoic lines, and dots are seen due to hair within the liquefied component.
Acoustic shadowing from a hyperechoic component and floating hyperechoic spherical structures are seen.
However, terms like “tip of the iceberg,” “Rokitansky nodule,” “dermoid mesh,” “dot-dash,” and “dermoid balls” are not preferred descriptors in the lexicon.
Hemorrhagic Cyst
Reticular patterns with fine, thin, lace-like lines due to fibrin strands and a retracting clot with avascular echogenic component showing angular, straight, or concave margins are seen.
Para-ovarian Cyst
A simple cyst existing separately from the ovary, which moves independently of the ovary when pressure is applied by the transducer, is seen.
It includes both para-ovarian and paratubal origin.
Hydrosalpinx
It has an incomplete septation due to the tubular nature of the lesion when visualized along an oblique plane.
It is substantially longer in one dimension than in the two perpendicular dimensions and may have endosalpingeal folds typically < 3 mm in height.
Peritoneal Inclusion Cyst
A cystic lesion with the ovary either at the margin or suspended within the lesion.
It follows the contour of the adjacent pelvic organs, contains septations, and does not exert a mass effect.
Usually occurs in patients with a history of post-surgical or post-inflammatory status in the pelvis.
SONOGRAPHIC CHARACTERISTICS OF OVARIAN OR ADNEXAL LESIONS (ORADS 3–5)
Ultrasound is often the first line of investigation, especially for distinguishing cystic from complex cystic solid and solid masses.
CYSTIC LESIONS
The characteristics to be considered are size, locularity, margin, solid component, external contour (smooth, irregular), internal contents, papillary projections, vascularity, and extra ovarian findings, as explained by ORADS Lexicon.6,7
Size
• It is best to use the single greatest diameter for risk stratification. The commonly used size cut-off is 3 cm and 10 cm.
• The average linear dimension (L+W+H/3) should be used for interval changes [Figure 4].
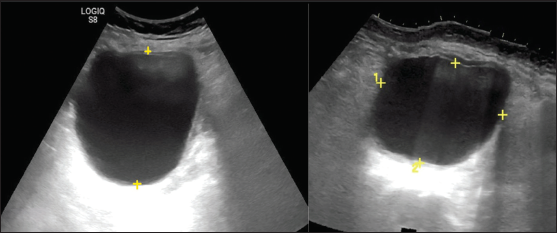
-
Ultrasonography images show unilocular cystic adnexal lesion. The average of linear dimension in three planes is used for interval change and the largest dimension for risk stratification.
Locularity
The lesion is classified as
1) Unilocular – Cystic lesion that contains a single compartment. It may contain 3 1 incomplete septum, wall irregularity < 3 mm height, or internal echoes.
2) Bilocular – Two locules and one complete septation.
3) Multilocular – a cyst with two or more complete septations resulting in three or more locules [Figure 5].
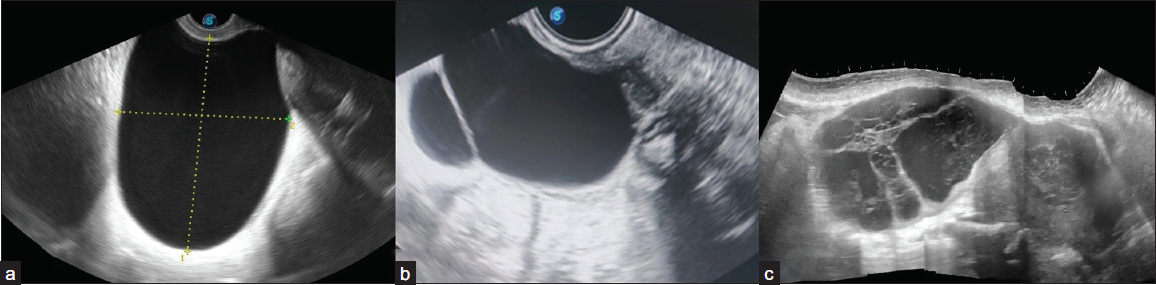
- Ultrasonography (USG) images of cystic adnexal lesions of three different patients show types of locularity. (a) Unilocular, (b) Bilocular, (c) Multilocular
Margins
1) Smooth – Regular, uniform throughout
2) Irregular – Non-uniform, focal thickening of < 3 mm, papillary projections or mural nodules, irregular incomplete septa [Figures 6a and 6b]
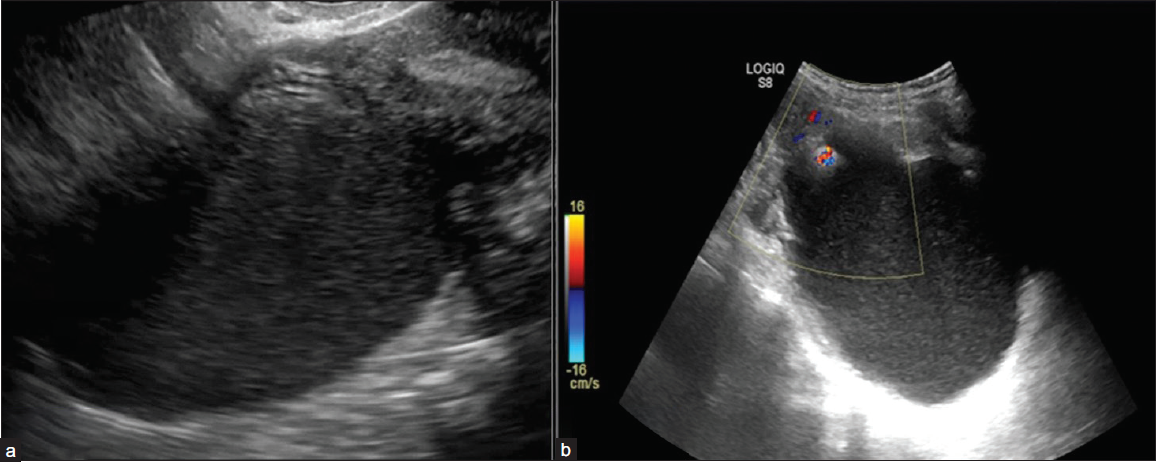
- Ultrasonography (USG) images depicting inner margins or walls of cystic lesions. (a) USG image shows a unilocular cyst with internal echoes and a smooth inner wall margin. (b) USG image shows a unilocular margin with an irregular inner margin and non-simple fluid in the form of internal echoes.
Solid component
2. Papillary projection- Focal wall thickening or solid tissue arising from cyst wall/septation that protrudes into cyst cavity 3 3 mm in height and is surrounded by fluid on three sides. Number important for risk stratification (< 4 vs. 3 4)
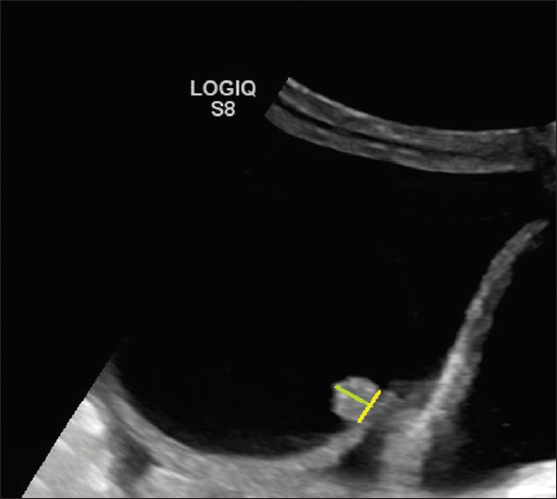
- Ultrasonography (USG) image shows a cystic lesion with a solid papillary projection. The height (green line) of the echogenic component has to be measured. If it measures 3 3 mm, then considered a solid component if it measures < 3 mm in height then considered as part of the irregularity of the wall.
Some intracystic components can appear solid on imaging and are categorized as “solid appearing lesions”:
• Hemorrhagic products – Initially, the hemorrhagic products appear in a lace-like reticular (fish net) pattern; once the fibrin molecules contract within the bloody component, the margins retract internally and appear sharp or concave as seen in our patient, in contrast to the papillary projections that appear cauliflower-like [Figure 8].
• Mucinous material – Sometimes lesions with mucinous content may show homogeneous ground glass echoes, giving a pseudo solid appearance with no evidence of internal vascularity.
• Fat material in the form of hyperechoic floating balls.
• Avascular hyperechoic structure with acoustic shadowing as in dermoid commonly known as Rokitansky nodule. [Figure 3b].
• Septa determines the locularity of the lesion and is not considered a solid component, irrespective of its thickness9
• Normal ovarian tissue should not be considered a solid component.
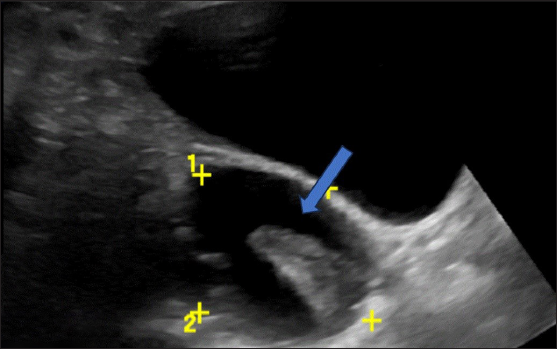
- Ultrasonography (USG) image shows a typical hemorrhagic cyst. A unilocular cyst with a retracted clot showing an acute margin appearing as avascular tissue (blue arrow).
Vascularity
Color score (CS) on Doppler ultrasonography is a numeric categorization (1–4) to characterize vascularity within a lesion as follows [Figure 9]:
1. No flow;
2. Minimal flow;
3. Moderate flow;
4. Very strong flow
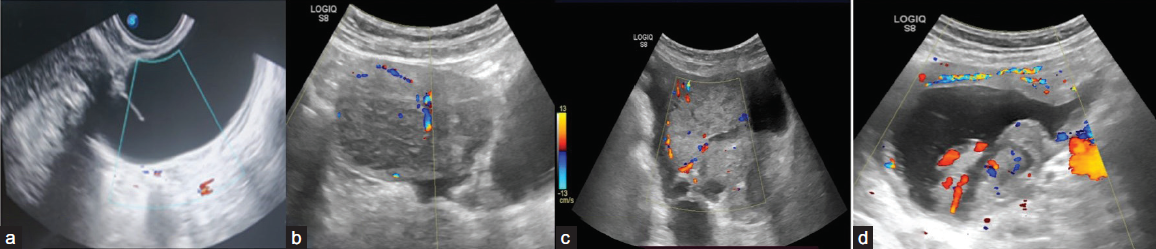
- Ultrasonography (USG) images of various adnexal lesions with different color score patterns: (a)-color score 1, (b) color score 2, (c) color score 3, (d) color score 4
Figure 10 shows the algorithmic approach to a cystic lesion and ORADS categorization.
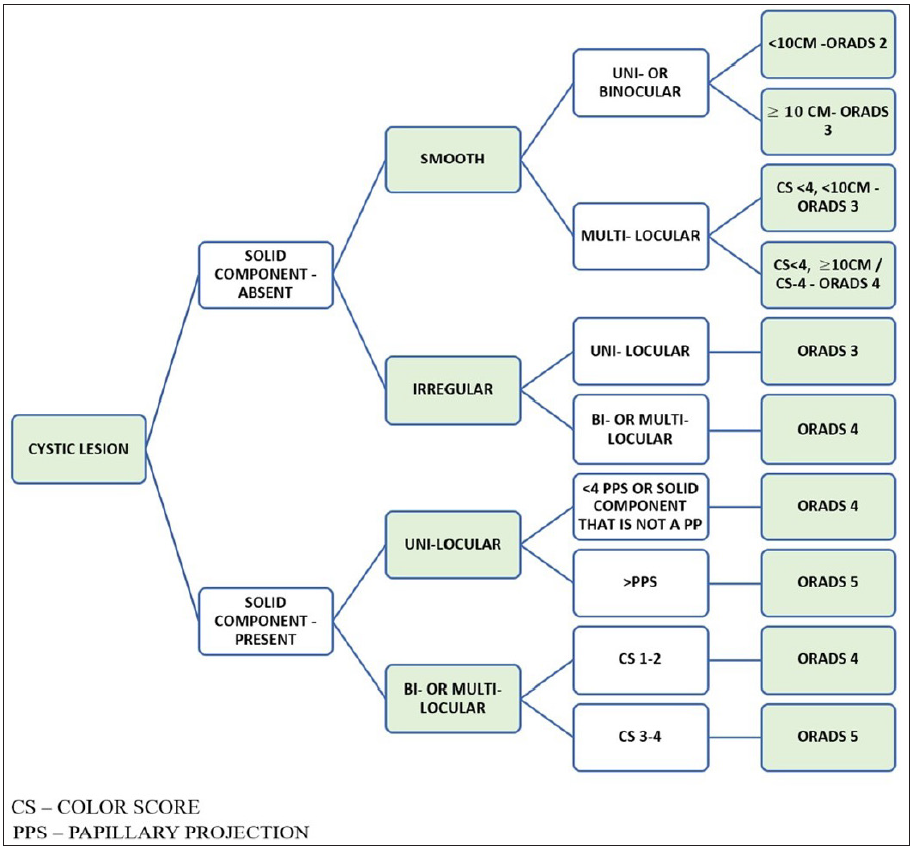
- Algorithmic approach to assign Ultrasound-Ovarian Adnexal Reporting and Data System (US-ORADS) for a cystic ovarian lesion.
SOLID LESIONS
Lesions where the solid components comprise 80% or more are categorized as solid lesions. The solid lesions should be assessed in a two-dimensional section.4,8
A purely solid lesion is 100% solid with no cystic components.9
Solid lesions with shadowing, smooth outer contour, and CS <4 is O-RADS 3. Solid lesions with a smooth outer contour and shadowing suggest fibromatous elements with a risk of malignancy <10 %, that is, ORADS-3.
All solid lesions with a CS 4 (very strong flow) or irregular outer contour remain in O-RADS 5 (high risk), regardless of the presence of shadowing [Figure 11].
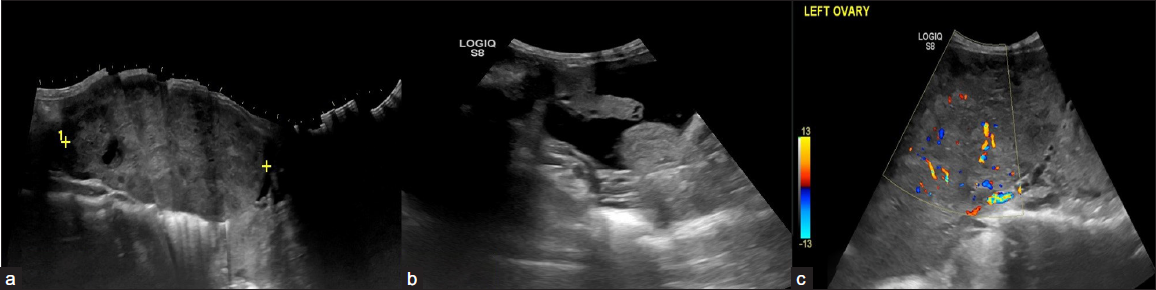
- A 23-year-old female with complaints of abdominal distension for 3 months. CA-125 level – 650 U/ml. On USG, A relatively defined multi-lobulated hypoechoic solid lesion with lobular margins in the left adnexa (a) with mild ascites (b), and left pleural effusion. (c) On Doppler the color score is 3. The above features are suggestive of the Ovarian-Adnexal Reporting and Data System ORADS 5 lesion. On histopathology, the above lesion is suggestive of dysgerminoma.
Figure 12 shows the algorithmic approach to a solid lesion and ORADS categorization.
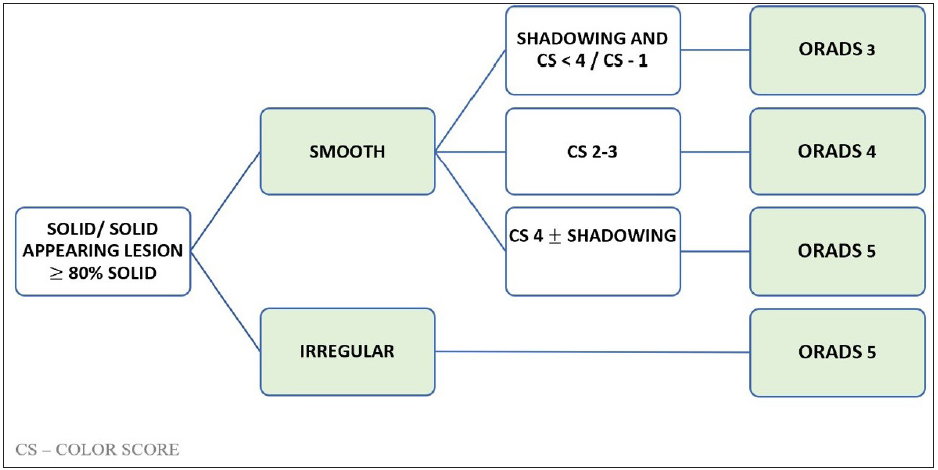
- Algorithmic approach to assign Ultrasound Ovarian-Adnexal Reporting and Data System US-ORADS for a solid ovarian lesion
Shadowing
Including shadowing as a descriptor improves the specificity of solid lesions when the outer contour is smooth, as shadowing suggests fibromatous elements. However, all solid lesions with a CS 4 (very strong flow) or irregular outer contour remain as O-RADS 5 (high risk), regardless of the presence of shadowing.
PITFALLS
Papillary Projection and Color Doppler
The difficulty of classifying unilocular adnexal cysts with papillary projections as benign or malignant using subjective assessment in comparison to other types of tumors has been confirmed by Valentin, Ameye, Savelli, et al.(2013).10
The mere presence of the papillary projection is not specific to the malignancy. The number of papillary projections should be counted. It is seen with few benign and borderline cystadenoma.
One of the cases includes chronic torsion and detorsion features with the presence of papillary projection [Figure 13].
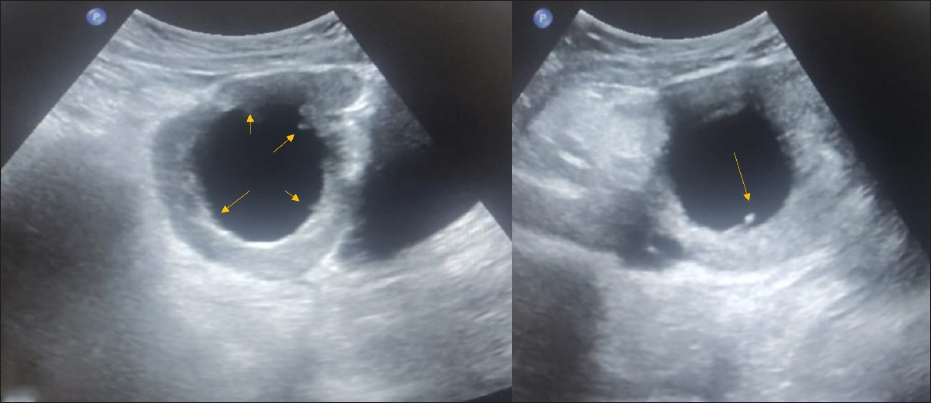
- An 18-year-old female with complaints of abdominal pain for ten days associated with nausea and vomiting. CA-125 level was normal. On USG abdomen– Unilocular cyst of size 6.5 × 6.6 cm noted in the right adnexa with an irregular inner wall and echogenic papillary projection at least 5 in number (arrows), not showing any vascularity on color doppler- ORADS 4. On MRI, pelvis features were suggestive of ovarian torsion.
PERITONEAL THICKENING AND NODULES
Peritoneal nodules observed in low- or intermediate-risk (O-RADS 3 or 4) adnexal lesions result in an upgraded risk assessment to O-RADS 5. The presence of peritoneal nodules is associated with conditions such as malignant, inflammatory, or infectious causes (like mucinous gastrointestinal malignancies or tuberculosis) that may share similar appearances; it is prudent to attribute the findings to the adnexal process in the absence of evidence suggesting alternative causes [Figure 14].11
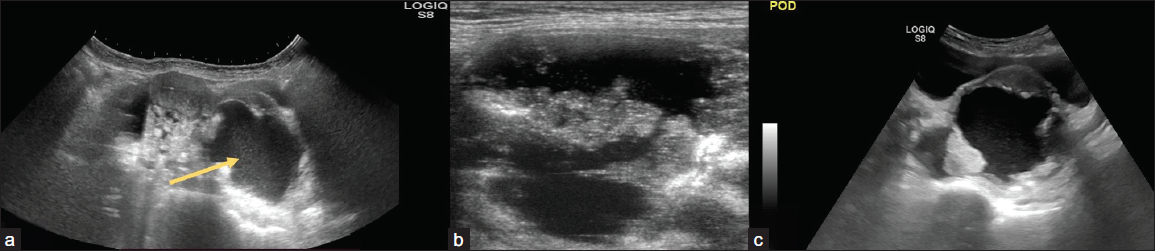
- (a) 31-year-old female with pain in her lower abdomen for one month. On USG abdomen cystic lesion (arrow) with heterogeneously hypoechoic solid lesion with moderate free fluid peritoneal cavity and (b, c) nodularity and fluid in the pouch of Douglas; Ovarian-Adnexal Reporting and Data System OARDS 5. On histopathological correlation – high-grade serous adenocarcinoma with peritoneal deposits.
ASCITES
In premenopausal women, fluid confined to the pouch of Douglas (space posterior to the uterus and between the rectum and uterus) may be considered physiologic. However, fluid that exceeds this amount is defined as ascites when it extends superior to the uterine fundus if the uterus is anteverted or anteflexed.
If the uterus is retroverted or retroflexed, fluid superior and anterior to the uterus (between the bladder and the uterus) is considered ascites.
When ascites are unexplained (e.g., cannot be attributed to a systemic or non-malignant adnexal cause), a lesion otherwise assessed O-RADS 3 or 4 should be upgraded to O-RADS 5. However, if the lesion is O-RADS 1 or 2, other plausible causes, such as fluid overload, renal impairment, and cirrhosis, should be considered. The report may describe the internal content of ascites, but this does not affect risk stratification.
Solid vs. Cystic Material
Solid tissue is confirmed by the presence of color Doppler flow, while the absence of flow may indicate debris or necrotic tissue, which provides less diagnostic value.
A helpful technique to differentiate solid material from debris is to apply gentle pressure with the transducer on the area of concern; if internal movement or a “jiggle” sign is observed, it suggests the presence of debris like blood clots. When there is uncertainty, it is advisable to classify the lesion as solid appearing [Figure 15].
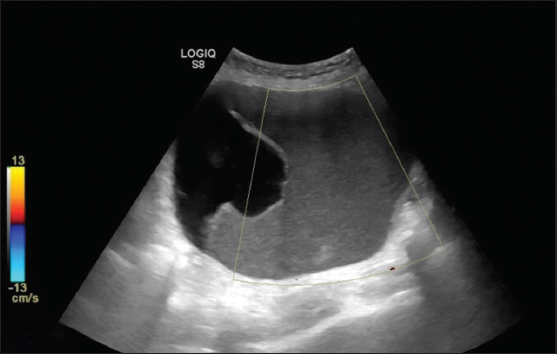
- Ultrasonography (USG) images show a unilocular cystic lesion with homogeneous ground glass echoes, giving a pseudo-solid appearance with no evidence of internal vascularity. Histopathology is suggestive of mucinous cystadenoma.
Eccentric Solid Tissue
To determine the distinction between an eccentric ovarian lesion and the ovary, the sliding test can be done by applying pressure with the transducer probe to see if the structure can be separated from the ovary. In some cases, bimanual pressure, with one hand on the abdomen and the probe positioned appropriately, may be necessary to confirm whether the lesion is ovarian or para-ovarian. When dealing with a solid eccentric lesion, careful examination for connecting tissue and vessels leading to the uterus aids in distinguishing it from an exophytic subserosal leiomyoma.
ROLE OF MRI ORADS
O-RADS MRI plays a crucial role in characterizing and assessing the risk of indeterminate adnexal lesions alongside ultrasound. O-RADS MRI is commonly utilized to evaluate lesions categorized as O-RADS 3 or 4 in the ultrasound. The O-RADS MRI offers higher negative predictive value (NPV) and positive predictive value (PPV) for malignancy due to the improved soft-tissue contrast provided by MRI.12
CONCLUSION
O-RADS US serves as a tool to simplify the characterization of adnexal lesions, minimize the use of misleading terminology, and aid in the management of such lesions. An algorithmic approach streamlines the assessment process. While prospective validation trials are necessary, the early adoption and concise reporting using the lexicon terminology promise to standardize language, reduce unnecessary surgeries, and establish an effective referral system for gynecologic oncologists.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient’s consent not required as patients identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Consensus document for management of epithelial ovarian cancer prepared as an outcome of ICMR subcommittee on epithelial ovarian cancer. Available as https://main.icmr.nic.in/sites/default/files/guidelines/Consensus
- The abnormal ovary: evolving concepts in diagnosis and management. Obstet Gynecol Clin. 2019;46:607-24.
- [Google Scholar]
- Comparison of O-RADS, GI-RADS, and IOTA simple rules regarding malignancy rate, validity, and reliability for diagnosis of adnexal masses. Eur Radiol. 2021;31:674-84.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Predicting the risk of malignancy in adnexal masses based on the Simple Rules from the International Ovarian Tumor Analysis group. Am J Obstet Gynecol. 2016;214:424-37.
- [CrossRef] [PubMed] [Google Scholar]
- The ovarian/adnexal reporting and data system for ultrasound: from standardized terminology to optimal risk assessment and management. Can Assoc Radiol J.. 2023;74:44-57.
- [CrossRef] [PubMed] [Google Scholar]
- O-RADS for ultrasound: a user’s guide, from the AJR special series on radiology reporting and data systems. Am J Roentgenol. 2021;216:1150-65.
- [Google Scholar]
- Ovarian-adnexal reporting lexicon for ultrasound: A white paper of the ACR Ovarian-Adnexal Reporting and Data System Committee. J Am Coll Radiol. 2018;15:1415-29.
- [CrossRef] [PubMed] [Google Scholar]
- Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet Gynecol. 2000;16:500-5.
- [CrossRef] [PubMed] [Google Scholar]
- O-RADS™ Ultrasound v2022 Lexicon Categories, Terms, and Definitions Revised: January 2023 https://www.acr.org/-/media/ACR/Files/RADS/O-RADS/O-RADS-US-v2022-Lexicon-Terms-Table-2023.pdf
- Unilocular adnexal cysts with papillary projections but no other solid components: Is there a diagnostic method that can classify them reliably as benign or malignant before surgery? Ultrasound Obstet Gynecol. 2013;41:570-81.
- [CrossRef] [PubMed] [Google Scholar]
- Ultrasound evaluation of ovarian masses and assessment of the extension of ovarian malignancy. Br J Radiol. 2021;94:20201375.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ovarian-Adnexal Reporting Data System Magnetic Resonance Imaging (O-RADS MRI) score for risk stratification of sonographically indeterminate adnexal masses. JAMA network open. 2020;3:e1919896.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]







