Translate this page into:
Prescription pattern of usage of analgesics in pain relief in cancer patients at a tertiary care teaching hospital - An observational prospective study
*Corresponding author: Dr. Ram Kumar Nagarajan, MD, Junior Resident, Department of Pharmacology, AIIMS, Bhopal, India. ram154700@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Nagarajan RK, Najmi A, Das S, Jain A, Kaore SN, Atal S, et al. Prescription pattern of usage of analgesics in pain relief in cancer patients at a tertiary care teaching hospital- An observational prospective study. Future Health. 2024;2:120-6. doi: 10.25259/FH_53_2024
Abstract
Objective
The World Health Organization has developed guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents to provide evidence-based guidance to healthcare providers on the adequate relief of pain associated with cancer. This prospective study was done to evaluate the prescription pattern of analgesics for the treatment of cancer pain.
Material and Methods
This prospective observational study was conducted in cancer patients attending the pain clinic of the Anesthesia and Radiotherapy Outpatient Department. Approval was obtained from the Institutional Human Ethics Committee. Inclusion criteria were age ≥18 years of either gender, willing to provide written informed consent, and patients undergoing radiotherapy, chemotherapy, or palliative treatment. Exclusion criteria were patients having psychiatric illness, not willing to provide written informed consent, patients having renal and or hepatic dysfunction, patients with comorbidities with chronic pain, and patients who underwent surgical intervention.
Results
A total of 100 participants were recruited during the study period of July 2022–April 2024 whose data were assessed. Participants were prescribed different classes of analgesic drugs, which included 30% participants on opioid analgesics, 30% on non-opioid analgesics, and 40% on combination therapy. Among opioid analgesics, morphine was the most commonly prescribed. Among non-opioid, paracetamol was the most commonly prescribed. Among combination therapy, tramadol was the most commonly prescribed. Pregabalin was most commonly used as an adjuvant analgesic.
Conclusion
This study also emphasizes on need for adequate treatment of cancer pain and vigilant monitoring of patients on opioids to prevent drug abuse, drug dependence, and adverse drug reactions.
Keywords
Cancer pain
Prescription pattern
Analgesics
Opioids
Introduction
Pain is an unpleasant sensory and emotional experience with actual or potential tissue damage or described in terms of such damage.1 The World Health Organization (WHO) has developed guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents to provide evidence-based guidance to healthcare providers on the adequate relief of pain associated with cancer. The ultimate goal of cancer pain management is to relieve pain to a level that allows for an acceptable quality of life. Recent guidelines by WHO in the year of 2019 are evidence-based guidelines developed using standard quality-assured methods. WHO mainly focuses on three areas, namely ‘Analgesia of cancer pain’, ‘adjuvant medicines for cancer pain’, and ‘management of pain related to bone metastases’. WHO Three-Step Analgesic Ladder, released in 1986, is a general guide to pain management. It guides us in managing pain based on its severity. Step 1 of this ladder is for mild pain management, which consists of non-opioid analgesics. Step 2 is for moderate type of pain management with weak opioids. Severe pain is managed with stronger opioids according to step 3 of the Analgesic Ladder. In all three steps, adjuvants can be added.2 Adjuvants include steroids, antidepressants, anticonvulsants, and bisphosphonates. Non-opioid analgesics include non-steroidal anti-inflammatory drugs and paracetamol. Weak opioids include codeine, tramadol, and morphine in lower doses. Strong opioids used for pain management are morphine in higher doses, fentanyl, oxycodone, and buprenorphine. WHO also suggests that the administration of analgesics ‘By Mouth’, ‘By the Clock’, ‘For the Individual’, and with ‘Attention to the details’, which means analgesics should be given through the oral route whenever possible. Doses of the analgesics should be given at fixed intervals of time appropriate for the patient. Analgesics should be chosen according to the individual patient’s type, severity, and cause of the pain, and the first and last doses of the day should be linked to the patient’s waking time and bedtime. The first step in pain management is a thorough clinical assessment of the patient and diagnosis of the underlying cause.2 The prevalence of pain in cancer patients ranges from 33% in patients after curative treatment to 59% in patients undergoing anticancer treatment and 64% in patients with advanced stages of the disease. Approximately 5%–10% of cancer survivors have chronic severe pain that interferes with normal day-to-day activities significantly.3
The objective of this study was to evaluate the pattern of usage and identify the proportion and frequency of analgesics and non-analgesic concomitant medications other than the chemotherapeutic agents for a period of 18 months in patients attending the pain clinic of the Anesthesia and Radiotherapy Outpatient Department.
MATERIAL and METHODS
This prospective observational study was conducted in cancer patients attending the pain clinic of the Anesthesia and Radiotherapy Outpatient Department. Approval was obtained from the Institutional Human Ethics Committee (IHEC). Inclusion criteria were age ≥18 years of either gender, willing to provide written informed consent, patients undergoing radiotherapy, chemotherapy, or palliative treatment. Exclusion criteria were patients having psychiatric illness, not willing to provide a written informed consent, patients having renal and/or hepatic dysfunction, patients with comorbidities with chronic pain, and patients who underwent surgical intervention. Baseline data were collected at Day 0 on sociodemographic - age, sex, education, family background, cancer history - location, grade and type, metastasis or localized, medical history - chemotherapy, radiotherapy, hormone therapy, immunotherapy, analgesics and adjuvants, surgical history, comorbid conditions, and prescription details including drug name, generic or branded, drug dosage, frequency of dosage, duration of treatment, and route of administration and pain history - presence, onset, character, location, duration, intensity. Follow-up data were collected on any change in prescription in terms of dosage adjusted/drugs stopped/drugs switched/drugs added on Day 28. Data collected from the patients were entered in data collection sheets (case record forms) and safely secured under the custody of the investigators The Study flow chart shows how the data were collected and analyzed..
Study flow chart
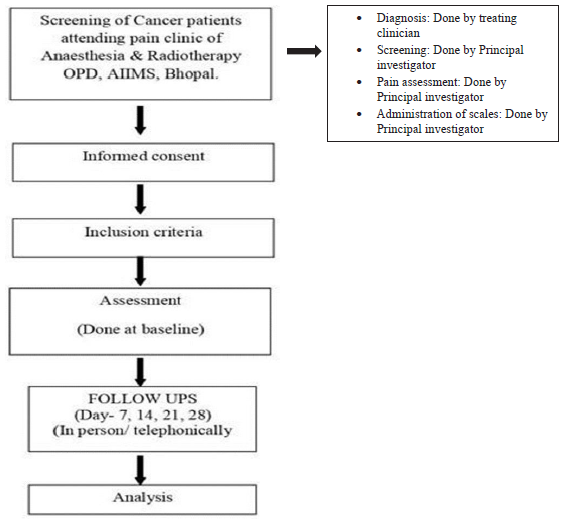
Statistical analysis
All the data collected during the study were recorded on the case record form and then was tabulated in Microsoft Excel. The data were analyzed using statistical software package SPSS version 26.0. Categorical variables such as gender, classes of drugs, responders, and non-responders were calculated using frequencies and proportions. Continuous variables like age, weight, and height were calculated using mean with standard deviation.
RESULTS
A total of 100 participants were recruited during the study period of July 2022–April 2024, whose data were assessed. Out of the 100 participants in the study, 48% were male and 52% were female [Figure 1]. The mean age of the participants was 48.50 years (SD: ±13.80 years) [Figure 2]. Out of the 100 participants, 26% fell in the age group of 18–40 years and 55% were in the age group of 41–60 years of age, and 19% greater than 60 years. The participants had a mean body weight of 56.95 kg (SD: ±8.59) and a mean height of 164.50 cm (SD: ±8.66). Table 1 shows the demographic details of the participants.

- Distribution of gender.

- Age distribution.
| S. No | Characteristics | Percentage |
|---|---|---|
| 2. | Age 18–40 years | 26% |
| 3. | Age 41–60 years | 55% |
| 4. | Age >60 years | 19% |
Prescription patterns of drugs used by the participants were characterized to comprehend the pattern of drug usage. Among all the classes of drugs, a combination of opioid and non-opioid analgesics was the maximum prescribed class (n=40) and among combination drug regimens, tramadol was the maximum prescribed drug (n=38). All the drugs were given by oral route for variable durations, and at follow-up, none of the drugs were withdrawn or replaced, nor any dose adjustment was done. Table 2 mentions the medication details with different classes of drugs prescribed, along with the individual drugs prescribed under each category. Table 3 shows the details of the adjuvant drugs prescribed. Figure 3 shows the different classes of drugs prescribed.
| S No. | Analgesics prescribed | Number of participants | Dose form | Dose (mg) | Dosage frequency | Frequency of prescription |
|---|---|---|---|---|---|---|
| 1. | Morphine | 22 | Tablet | 5 mg | TDS | 16 |
| QID | 05 | |||||
| Q4H | 01 | |||||
| 2. | Methadone | 01 | Syrup | 12.5 mg | BD | 01 |
| 3. | Tramadol | 03 | Tablet | 50 mg | BD | 01 |
| 50 mg | TDS | 02 | ||||
| 4. | Tapentadol | 02 | Tablet | 50 mg | BD | 02 |
| 5. | Paracetamol | 17 | Tablet | 500 mg | BD | 10 |
| 500 mg | TDS | 03 | ||||
| 650 mg | BD | 04 | ||||
| 6. | Ibuprofen | 03 | Tablet | 400 mg | BD | 03 |
| 7. | Ketorolac | 01 | Tablet | 10 mg | BD | 01 |
| 8. | Diclofenac | 01 | Tablet | 50 mg | BD | 01 |
| 9. | Aceclofenac+Paracetamol | 09 | Tablet | 100 mg+325 mg | BD | 09 |
| 10. | Tramadol+Paracetamol | 38 | Tablet | 37.5 mg+325 mg | BD | 39 |
| 11. | Morphine+Ibuprofen | 01 | Tablet | 5 mg+400 mg | TDS | 01 |
| 12. | Tramadol+Etoricoxib | 01 | Tablet | 50 mg+60 mg | BD | 01 |
TDS: Thrice daily, QID: Four times a day, Q4H: Every 4 hours, BD: Twice daily.
| S No. | Adjuvants prescribed | No of participants | Dose form | Dose (mg) | Dosage frequency | Frequency of prescription |
|---|---|---|---|---|---|---|
| 1. | Pregabalin | 05 | Tablet | 75 mg | HS | 04 |
| 150 mg | HS | 01 | ||||
| 2. | Dexamethasone | 04 | Tablet | 4 mg | BD | 03 |
| 8 mg | BD | 01 | ||||
| 3. | Drotaverine | 01 | Tablet | 80 mg | OD | 01 |
| 4. | Prednisolone | 01 | Tablet | 10 mg | OD | 01 |
| 5. | Nortriptyline | 03 | Tablet | 10 mg | HS | 03 |
| 6. | Gabapentin | 02 | Tablet | 100 mg | HS | 02 |
| 7. | Baclofen | 02 | Tablet | 10 mg | BD | 02 |
| 8. | Amitriptyline | 01 | Tablet | 25 mg | HS | 01 |
| 9. | Mefenamic acid | 02 | Tablet | 500 mg | BD | 02 |
HS: at bedtime, BD: Twice daily, OD: Once daily.

- Classes of drugs prescribed.
DISCUSSION
This study was conducted at the radiotherapy OPD and pain clinic OPD of AIIMS, Bhopal, in collaboration with the Department of Pharmacology, Department of Radiotherapy, and Department of Anesthesia. In this prospective observational study, 100 patients with documented diagnoses of cancer presenting to the radiotherapy and pain clinic OPD with pain were recruited after taking their informed written consent. Cancer pain can be defined as pain caused by the primary cancer itself or metastases or its treatment. The prevalence of chronic pain is about 30%–50% among patients with active cancer and 70%–90% among those with advanced disease.2
The average age of the participants in this study was around 48.5 years, with most participants in the age group of 41–60 years (55%). It is well-known that the cumulative risk for all cancers combined increases with age, up to age 70 years, then decreases slightly, which is in concordance with this study that there were a smaller number of cases in participants under 40 years of age and a greater number of cases in participants above 40 years of age.4 The reason for the dip in the incidence of cancer in people above 70 years of age is due to the decline in the lifespan of people as their age increases. One of the main causes of death across the globe is cancer. When it comes to the incidence, prognosis, and mortality of certain cancers, gender is a significant factor. Men die from cancer at a rate 40% greater than that of women, and men are more likely to have cancer than women. The incidence of cancer in the male gender is 20% higher than in females.5 A slight difference in the gender distribution of cancer was established in this study that 52% of the participants were female and 48% were male.
According to a systematic review, the majority of opioid usage has involved individuals with moderate-to-severe cancer pain. Although they have well-documented adverse effects, opioids are an effective pain reliever in this population. Unless there is a contraindication, patients with moderate-to-severe pain associated with cancer or active cancer treatment should be provided opioids. To achieve satisfactory analgesia and patient goals, opioids should be started PRN (as needed) at the lowest possible dose, with periodic titration and early assessment. To date, morphine is the most commonly prescribed drug for cancer pain management.6 In the subgroup analysis, we found that when given as a monotherapy, among the opioid analgesic-prescribed patients, morphine was prescribed to the majority of participants (22/30; 73.33%), followed by tramadol to five participants (5/30; 16.67%). However, in combination therapy, tramadol was the most commonly prescribed opioid in combination with paracetamol given to most participants (38/40; 95%). To varying degrees, systemically administered alpha-2 adrenoreceptor agonists (clonidine and dexmedetomidine), gabapentinoids (gabapentin and pregabalin), N-methyl-d-aspartate receptor antagonists (ketamine and magnesium), lidocaine, and dexamethasone can reduce pain intensity. Adjuvants can also lessen the negative effects associated with opioids, although their usage may be limited by additional side effects they may produce. Their impact on opioid usage and pain scores varies. The best ways to administer these adjuvant drugs systemically and the therapeutic implications of the decreased opioid intake and pain severity have not yet been established. They have not yet gained widespread acceptance for their routine usage in multimodal analgesia, and it is unknown how they will affect the pain intensity.7 The adjuvant drugs prescribed in this study were pregabalin, mefenamic acid, drotaverine, prednisolone, nortriptyline, gabapentin, baclofen, amitriptyline, and dexamethasone. Among these adjuvant drugs, pregabalin was the most commonly used drug. Adjuvant medications mostly consist of corticosteroids, antidepressants, and antiepileptics. Due to their unique chemical make-up, these medications should not be prescribed frequently. When used for neuropathic pain in non-cancer diseases, antidepressant or antiepileptic medications have been shown to boost pain relief when combined with opioids. There is not enough data, though, to suggest that they are helpful in treating cancer pain. Physicians should weigh the higher risk of such combination therapy’s negative effects against the limited chance of benefit for patients experiencing pain associated with tumors in cancer. Patients who do not improve their pain management but still feel depressed may benefit from taking an antidepressant. For people who are really anxious, an anxiolytic may be utilized.
The different classes of drugs prescribed in this study were in accordance with the well-established WHO guidelines for cancer pain.2 The results of this study were also in congruence with the above-mentioned studies as well as with a recent observational study done by Lawati et al., where they analyzed the prescription pattern, wherein the usage of analgesics was similar to this study.8
For certain cancer pain types, corticosteroids are advised by a number of current guidelines, especially when the pain is associated with inflammation and edema. Only a few situations can benefit from the use of corticosteroids, anticonvulsants, and neuroleptic medications.9 While acknowledging the paucity of evidence to support its habitual usage, several recommendations still allow for the choice to take ketamine. While there is little evidence to suggest that intravenous lidocaine can decrease pain intensity in certain patients, another research has shown that it is ineffective. Because there is a chance of frequent side effects when using lidocaine, professional supervision is required. Thus, lidocaine may be a viable treatment choice for cancer pain that is not responsive to opioids. Tetrahydrocannabinol (THC) has been shown to amplify the antinociceptive impact of morphine in preclinical testing. Additionally, early research has suggested that THC may have a role as an adjuvant treatment for pain in cancer patients. It is unclear whether there is a benefit. Thus more research in people with moderate to severe cancer pain is required. The usage of opioid drugs has grown significantly over the last 20 years, which has raised the risk of drug abuse and misuse, as well as opioid dependence and deaths associated with it. In a pharmacovigilance study done by Chiappini et al. to ascertain the presence and type of pharmacovigilance signals related to abuse, misuse, and dependence on various prescription opioids like pentazocine, oxycodone, fentanyl, codeine, and dihydrocodeine, the authors have examined the FDA Adverse Events Reporting System (FAERS) and Eudra Vigilance (EV) pharmacovigilance databases to find and characterize potential abuse, misuse, and dependence-related problems10. Pharmacovigilance signal measures (i.e., reporting odds ratio, proportional reporting ratio, information component, and empirical Bayesian geometric mean) were computed for preferred terms (PTs) of abuse, misuse, dependence, and withdrawal, as well as PTs eventually related to them (e.g., aggression), after a descriptive analysis of the chosen adverse drug reactions (ADRs) were completed. In both datasets, there was a rise in ADR reports for the chosen opioids between 2003 and 2018.10 For the chosen opioids, EV received 16,506 and FAERS received 130,293 unique ADRs, respectively. Tramadol and oxycodone were more strongly linked to drug dependence and withdrawal than other opioids, although fentanyl and oxycodone were the opioids with the highest observed abuse concerns. The most often reported concurrent drug uses included benzodiazepines, antidepressants, other opioids, antihistamines, recreational drugs (including cocaine and alcohol), and a few novel psychoactive compounds like mitragynine and cathinone.10 ADR reports in pharmacovigilance databases, which should be regarded as a resource for tracking and preventing such concerns, validated the availability of data on prescription opioid abuse and dependence. When prescribing opioids, psychiatrists and other healthcare professionals should be mindful of the potential for abuse, dependency, and side effects, particularly when combined with other medications.
In an observational study regarding ADRs related to opioids by Pinilla-Monsalve et al., the authors recruited 3063 patients receiving opioids and found an altogether of 4437 problems related to opioid intake.11 Tramadol and morphine were the most commonly used opioids, according to that study. ADRs accounted for 93.15% were opioid-related problems, with 32.28% of those cases being severe. The authors also found that men were more likely to experience vascular, psychological, urinary, and hematological issues than women were, with women having proportionately more neurological and gastrointestinal diseases. The prognosis was linked to oral administration, age, weak opioids, and neurological–and cardiovascular reactions. In addition, 8.39% of these reactions did not resolve. Out of the 100 participants in the present study who had attended the follow-up visits, 11 participants presented with ADRs. The most commonly reported ADR was constipation (n=3), and in all those participants, morphine was prescribed. Constipation is a documented side effect of it. Other ADRs include nausea (n=2), drowsiness (n=2), vomiting (n=1), epigastric pain (n=1), rash (n=1), and bloating sensation (n=1). Drugs implicated in these ADRs were tramadol, morphine, paracetamol, ibuprofen, pregabalin, dexamethasone, baclofen, etoricoxib, and diclofenac, respectively. Causality analysis of all these reported ADRs was done using the WHO Uppsala monitoring scale.12 We found that out of 11 reported ADRs, most (10/11; 90.90%) belong to the possible category, and only 1 fell under the unlikely category.
In the clinical setting, morphine and other medications that agonistically interact with µ opioid receptors (MORs) are useful for treating pain as well as certain other conditions like cough and diarrhea. The availability of illicit heroin, fentanyl, and synthetic opioids has increased, and convergent events such as the overprescription of opioid analgesics and the increased abuse liability of MOR agonists have led to an increase in the incidence of opioid misuse and overdose death.13 The surge in opioid misuse has led to a renewed focus on creating novel treatments for opioid overdose and abuse as well as novel analgesics with lower potential for addiction. Preclinical assays that investigate the expression, determinants, and treatment of opioid effects related to abuse are a crucial component of research addressing these concerns. Prescription opioids are abused by a variety of methods, such as injection, nasal inhalation (also known as ‘snorting’), oral administration of intact drugs, and oral ingestion following product manipulation (e.g., crushing or chewing). Both immediate- and extended-release opioids are subject to manipulation for non-oral use, which aims to change the active ingredient into a more easily abused form (such as powder for nasal inhalation or solution for intravenous injection and release the opioid more quickly; a practice known as dose-dumping). Non-oral opioid delivery routes are associated with more severe medical consequences. Furthermore, tampering with prescription opioid medication usage is linked to higher medical costs than abuse without tampering. One element of a complex plan to lessen opioid misuse and abuse while preserving the supply of opioid drugs for qualified patients is the creation of abuse-deterrent formulations (ADFs) of opioid analgesics. Opioid abuse-deterrent opioids are designed to prevent manipulation or reduce the pleasure associated with abusing the altered product. Examples of these qualities include physical and chemical barriers and opioid agonist/antagonist combos. Even though these medicines would not completely stop misuse, the USFDA has actively promoted the development of ADFs for opioid analgesics with the claimed purpose of effectively preventing abuse.14
This was a prospective observational study, so it has all the limitations of such a study design, like confounding factors, biases, etc. Biases may include selection bias, recall bias, and confirmation bias. The small sample size was also a limiting factor of the study. Pain is a subjective component, and it differs from person to person. Pain is also qualitative data; assessing qualitative data with a quantitative scale itself has its own demerits.
CONCLUSION
This prospective study was conducted to evaluate the prescription pattern of analgesics for the treatment of cancer pain. Among opioid analgesics, morphine was the most commonly prescribed for cancer pain. Among non-opioids, paracetamol was the most commonly prescribed analgesic. Among combination therapy, tramadol and paracetamol were the most commonly prescribed analgesics. Pregabalin was the most commonly prescribed as an adjuvant analgesic. Moving forward, more disciplines dealing with cancer pain management to be included for robust data and framing adequate treatment; hence quality of life of cancer patients can be improved.
Ethical approval
The research/study was approved by the Institutional Review Board at AIIMS, BHOPAL, number IHEC-PGR/2022/PG/Jan/32, dated 12/09/2022.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of AI-assisted technology for assisting in the writing of the manuscript and no images were manipulated using AI.
References
- Terminology | International association for the study of pain [Internet]. International association for the study of pain (IASP). [cited 2022 Jul 11]. Available from: https://www.iasp-pain.org/resources/terminology/
- WHO guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents.
- Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2018;29:iv166-iv191.
- [CrossRef] [PubMed] [Google Scholar]
- Age and cancer risk: A potentially modifiable relationship. Am J Prev Med. 2014;46:S7-15.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Sex differences in cancer: Epidemiology, genetics and therapy. Biomol Ther (Seoul). 2018;26:335-42.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Use of opioids for adults with pain from cancer or cancer treatment: ASCO guideline. J Clin Oncol. 2023;41:914-30.
- [CrossRef] [PubMed] [Google Scholar]
- Systemic non-opioid adjuvant analgesics: Their role in acute postoperative pain in adults. Trends Anaesth Crit Care. 2014;4:10-8. Available from: https://doi.org/10.1016/j.tacc.2013.10.002
- [CrossRef] [Google Scholar]
- Prescription patterns of analgesic drugs in the management of pain among palliative care patients at a tertiary hospital in Oman: A Retrospective Observational Study. Cureus. 2023;15:e41501.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cancer pain and therapy. Acta Clin Croat. 2022;61:103-8.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Pharmacovigilance signals of the opioid epidemic over 10 years: Data mining methods in the analysis of pharmacovigilance datasets collecting adverse drug reactions (ADRs) reported to EudraVigilance (EV) and the FDA adverse event reporting system (FAERS) Pharmaceuticals (Basel). 2022;15:675.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- [Problems and adverse reactions related to opioid analgesics in Colombia] Rev Neurol. 2021;73:39-49.
- [CrossRef] [PubMed] [Google Scholar]
- Determinants of opioid abuse potential: Insights using intracranial self-stimulation. Peptides. 2019;112:23-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Abuse-deterrent opioid analgesics: A guide for clinicians. Pain Manag. 2020;10:55-62.
- [CrossRef] [PubMed] [Google Scholar]








