Translate this page into:
Comparison of collateral status in anterior circulation acute ischemic stroke on single-phase and multiphase CT angiography in prediction of clinical and radiological outcome
*Corresponding author: Radha Sarawagi, Department of Radiodiagnosis and Imaging, All India Institute of Medical Sciences (AIIMS), Bhopal, India. radha.radiodiagnosis@aiimsbhopal.edu.in
-
Received: ,
Accepted: ,
How to cite this article: Gachhi PR, Sarawagi R, Malik R, Kumar A, Rai N. Comparison of collateral status in anterior circulation acute ischemic stroke on single-phase and multiphase CT angiography in prediction of clinical and radiological outcome. Future Health. 2024;2:7–14. doi: 10.25259/FH_7_2024
Abstract
Objectives
Various collateral scoring systems are used to assess the collateral status on single or multiphase CT angiography (CTA) to forecast outcomes in anterior circulation acute ischemic stroke patients. Our study aimed to compare the predictive ability of these collateral scoring systems in determining clinical and radiological outcomes in patients with anterior circulation acute ischemic stroke.
Material and Methods
In this observational prospective study, data was obtained from December 2020 to June 2022 in consecutive acute ischemic stroke patients referred to our department for neuroimaging. The collateral scores were assessed using Tan and Menon scoring systems described for single and multiphase CTA, respectively. The correlations between various collateral scores, the National Institute of Health Stroke Scale (NIHSS), Modified Rankin Scale (mRS), and final infarct volume were studied. Receiver operating characteristics (ROC) curves and multivariate logistic regression analysis were performed to assess the predictive ability of scoring systems for clinical and radiological outcomes
Results
A total of 32 patients met the inclusion criteria. Both single- and multiphase collateral scores showed statistically significant negative correlations with clinical and radiological outcomes. Spearman correlation coefficient and p-value for the radiological outcome being -Tan score (r- -0.73, p- < 0.001), single-phase Menon score (r- -0.81, p- < 0.001), and multiphase Menon score (r- -0.79, p- < 0.001). ROC curve analysis revealed that the multiphase Menon score performed slightly better than the single-phase Menon score (area under the curve [AUC], 0.815 vs 0.810) in predicting a favorable 90-day modified Rankin scale score.
Conclusion
Our study concluded that collateral status on both multiphase CTA (mCT) and conventional (Single phase arterial CTA) showed statistically significant results in the prediction of clinical and radiological outcomes.
Keywords
Stroke
CT Angiography
Collaterals
Multiphase
INTRODUCTION
Stroke, a highly preventable and treatable condition, could lead to ∼10 million deaths annually by 2050, primarily affecting low and middle-income countries. This projection comes from the collaborative effort of the World Stroke Organization and the Lancet Neurology Commission. The report underscores that stroke deaths are expected to surge from 6.6 million in 2020 to a daunting 9.7 million by 2050.1 The burden of stroke is increasing in India, now being the fourth leading cause of death and the fifth leading cause of disability.2
Ischemic strokes can present in predetermined syndromes due to the effect of decreased blood flow to particular areas of the brain that correlate to exam findings, thus allowing the clinicians to be able to predict the location of brain vasculature that can be affected. The middle cerebral artery (MCA) is the most common artery involved in stroke.3
Neuroimaging of cerebrovascular status and hemodynamics has vastly improved our understanding of stroke mechanisms and provided information in therapeutic decision-making.
With the evolution of stroke therapies (like endovascular therapy) and the established impact of collateral circulation; imaging tools are crucial in recognizing patients who may benefit from specific treatment strategies.4
It has been shown that collaterals are essential in preserving cerebral perfusion in acute ischemic stroke due to large vessel occlusion and are relevant in the outcome prediction. Non-collateralized brain parenchyma quickly undergoes irreversible damage, forming an ischemic core, while the surrounding collateralized brain tissue, also referred to as the penumbra, is temporarily protected from infarction. The extent of collaterals thus determines the size of the ischemic core and penumbra. Various collateral scores have been published for middle cerebral artery territory by considering different features of cerebral collateralization. However, there is no gold standard for collateral assessment in CTA.5
The most commonly used tool for the evaluation of acute ischemic stroke in current clinical practice is multimodal CT, which includes non-contrast CT (NCCT), single-phase CT angiography (sCT), and CT perfusion (CTP). While NCCT allows rapid distinction of hemorrhagic from ischemic stroke, sCT can determine vascular occlusion and demonstrate collateral status. Furthermore, CTP can be used to define the ischemic core and at-risk tissue (penumbra) and indirectly reveal collateral circulation.4 Multiphase CTA (mCT) is a new technique that generates time-resolved temporal cerebral angiography of pial vessel filling. This technique is quick to perform, less vulnerable to patient motion, and yields images that are easy to interpret.
MATERIAL AND METHODS
Study Protocol
This observational, prospective study was conducted in a tertiary care hospital after getting necessary approvals from the Research Review Committee and Institutional Human Ethics Committee.
All adult patients with acute ischemic stroke referred for CT angiography to the Radiodiagnosis Department during the 18-month study period from December 2020 to June 2022 were included.
Informed consent was obtained from every patient before their recruitment. Exclusion Criteria were Posterior circulation stroke, intracranial hemorrhage, previous moderate to large stroke in the ipsilateral hemisphere, poor image quality due to motion artifact or incomplete acquisition, and one with no follow-up imaging.
Imaging technique
CTA examinations were performed using a dual-source 128-slice CT scanner (Somatom Definition Flash, Siemens Healthcare). All patients who fulfilled the inclusion criteria underwent standard unenhanced plain CT with 0.625-mm section thickness. CTA was triggered by bolus tracking, with the region of interest placed in the posterior aortic arch and, the trigger threshold set at 150 HU. Triple-phase CT angiograms of the brain vasculature were acquired. The area extending from the arch of the aorta to the vertex was covered during the peak arterial phase. The skull base and the vertex were the regions included in the peak and late venous phases, respectively. The angiograms were acquired at an interval of 8 seconds. A total of 50 mL of iodinated contrast material (iohexol/iodixanol) was injected at 5 mL/s, followed by 30 mL of the normal saline chase. The source images were reformatted into 3-mm thick axial, coronal, and sagittal projections.
Image analysis
In the multiphase technique, mCT collateral grading was done using a 5-point Menon score.6 Good collaterals are Grade 4 or above on Menon’s 5-point score. The first phase of mCT angiography performed as per the standard protocol was used for traditional sCT angiography. Single-phase Tan7 collateral grading (0–3) and single-phase Menon 5-point score 6 were assessed. Tan’s score of 32 and Menon’s score of 34 were good collaterals [Figures 1 and 2].
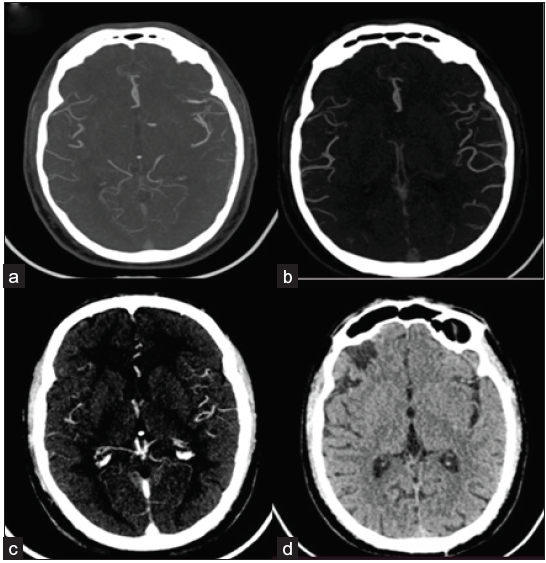
- (a) Axial MIP arterial, (b) peak-venous, and (c) venous images of multiphase CT angiography study of conservatively managed right frontal infarct in a 52-year-old male patient who presented with acute ischemic stroke revealed infarct collateral status of 3, 5 and 5 as per Tan, Menon (sCT) and Menon (mCT) scoring systems respectively. Follow-up axial CT image (d) revealed a final infarct volume of 2.1 cc. The final mRS of the patient was 1.
![(a) NCCT head axial image of a 70-year-old male patient on initial presentation revealed a dense MCA sign on the left side and subtle hypoattenuation & sulcal effacement of the ipsilateral cerebral hemisphere. (b) CT angiography revealed non-opacification of contrast in the M1 & M2 segment of left MCA on coronal MIP reformat image. (c) Axial sections of arterial and (d) venous phase images revealed collateral status of 1, 1, and 1, respectively, as per Tan, Menon (sCT), and Menon (mCT) scoring systems. (e) Follow-up axial CT image revealed hemorrhagic transformation. The final mRS of the patient was 6. [MCA: Middle Cerebral Artery, MIP: Maximum Intensity Projection]](/content/174/2024/2/1/img/FH_7_2024-g2.png)
- (a) NCCT head axial image of a 70-year-old male patient on initial presentation revealed a dense MCA sign on the left side and subtle hypoattenuation & sulcal effacement of the ipsilateral cerebral hemisphere. (b) CT angiography revealed non-opacification of contrast in the M1 & M2 segment of left MCA on coronal MIP reformat image. (c) Axial sections of arterial and (d) venous phase images revealed collateral status of 1, 1, and 1, respectively, as per Tan, Menon (sCT), and Menon (mCT) scoring systems. (e) Follow-up axial CT image revealed hemorrhagic transformation. The final mRS of the patient was 6. [MCA: Middle Cerebral Artery, MIP: Maximum Intensity Projection]
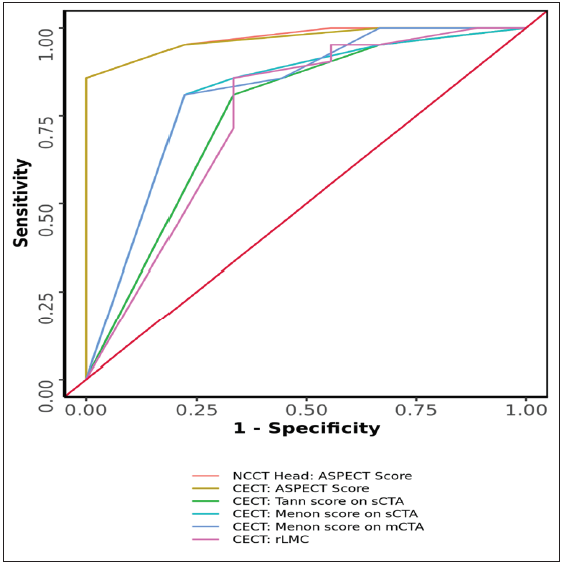
- Receiver operating characteristic (ROC) Curve Analysis Showing Diagnostic Performance, various predictors in Predicting outcome. NCCT: Non-contrast CT, CECT: Contrast enhanced CT, ASPECT: Alberta stroke program early CT score, sCTA: single-phase CT angiography, mCTA: multiphase CT angiography.
Alberta stroke program early CT score (ASPECTS) on NCCT images was also noted.8
Baseline NIHSS was evaluated and recorded by an experienced neurologist.9 Age, gender, risk factors, and type of treatment received (conservative management, intravenous thrombolysis) were documented. As per our institutional protocol, follow-up cross-sectional imaging was assessed for final infarct volume by voxel-based method and hemorrhagic transformation, if any. NIHSS score was also recorded at discharge. mRS was evaluated at admission, discharge, and at 28 and 90 days.10 The last follow-up mRS that could be obtained was considered for outcome assessment and labeled as the final mRS. Those with final mRS £ 2 were considered good functional outcomes, and those with > 2 were considered as poor functional outcomes.
STATISTICAL ANALYSIS
The statistical analysis was performed using IBM SPSS statistic software. Categorical variables are presented as frequency and percentage, whereas continuous variables are expressed as mean ± standard deviation, median & range and then analyzed accordingly. The Chi-square, Wilcoxon–Mann–Whitney U, and Fisher exact tests were used for categorical data. The Spearman correlation coefficient was used to analyze the association between the radiologic predictors and clinic-radiological outcome parameters. ROC analysis was performed to compare the prediction ability of different predictive parameters. All tests were performed at 95% confidence interval and 5% error, and a value of p £ 0.05 was considered statistically significant.
RESULTS
A total of 72 patients were referred to our department for acute stroke imaging. Of these, 41 (56.9%) were males, and 31 (43.1%) were females. 20 (28%) patients had a hemorrhagic stroke and were excluded from the study. Among the patients with non-hemorrhagic ischemic stroke, 9 had posterior circulation stroke & 8 patients had previous ipsilateral moderate-large volume infarct; they were excluded from the study. Two had both intracranial hemorrhage and posterior circulation stroke, and one patient had both posterior circulation stroke and a previous large-volume infarct. Four patients could not undergo the CTA study as they were unstable, and two patients had poor-quality images; hence, they were excluded. Thus, 32 patients finally met the inclusion criteria. Among these patients, only two patients were within the window period and received IV thrombolysis therapy. The remaining patients received conservative management.
Out of 32 patients, 20 were male, and 12 were female, with the mean age of presentation of anterior circulation stroke being 56.53 ± 14.28 years and 56.25 ± 19.26 years. Common risk factors were hypertension (63.3%) and diabetes mellitus (50%), followed by smoking (36.7%). However, among males, smoking and hypertension were the most common risk factors encountered [Table 1].
| Parameters | Clinical Outcome | p-value | |
|---|---|---|---|
| Good (n = 21) | Poor (n = 9) | ||
| Age (Years) | 51.10 ± 14.43 | 63.00 ± 17.82 | 0.0981 |
| Gender | 0.2492 | ||
| Male | 11 (52.4%) | 7 (77.8%) | |
| Female | 10 (47.6%) | 2 (22.2%) | |
| Risk Factors: | |||
| Smoking | 7 (33.3%) | 4 (44.4%) | 0.687 |
| Hypertension | 12 (57.1%) | 7 (77.8%) | 0.419 |
| Diabetes mellitus | 9 (42.9%) | 6 (66.7%) | 0.427 |
| Dyslipidemia | 3 (14.3%) | 0 (0.0%) | 0.534 |
| H/o stroke/TIA | 2 (9.5%) | 0 (0.0%) | 1.000 |
| Atrial fibrillation | 0 (0.0%) | 0 (0.0%) | 1.000 |
| Coronary artery disease | 1 (4.8%) | 2 (22.2%) | 0.207 |
| Chronic kidney disease | 0 (0.0%) | 1 (11.1%) | 0.300 |
| ASPECT Score*** | 9.00 ± 0.71 | 6.11 ± 1.76 | <0.0011 |
| Tann score on sCT*** | 2.76 ± 0.54 | 2.00 ± 0.87 | 0.0101 |
| Menon score on sCT*** | 4.62 ± 0.86 | 3.22 ± 1.20 | 0.0021 |
| Menon score on mCT*** | 4.67 ± 0.73 | 3.33 ± 1.22 | 0.0021 |
| NIHSS Score (Admission)*** | 4.29 ± 1.01 | 17.44 ± 5.22 | <0.0011 |
| Follow-up imaging: Final Infarct Volume (cc)*** | 6.48 ± 17.89 | 49.91 ± 39.78 | <0.0011 |
| Follow-up imaging: Hemorrhagic Transformation | 0 (0.0%) | 0 (0.0%) | 1.0003 |
***Significant at p < 0.05, 1: Wilcoxon-Mann-Whitney U Test, 2: Fisher’s Exact Test, 3: Chi-Squared Test, TIA: Transient ischemic attack, NIHSS: National Institute of Health Stroke Scale, ASPECT: Alberta stroke program early CT score, sCT: single-phase CT angiography, mCT: multiphase CT angiography.
The mean NIHSS score of patients with anterior circulation ischemic stroke at admission was 8.23 ± 6.77 (Range: 3-26). In correlation with the clinical outcome, the mean NIHSS score in patients with good clinical outcomes (i.e., functionally independent, final mRS £2) was 4.29 ± 1.01 and 3.81 ± 0.93 at admission and discharge, respectively. While 17.44 ± 5.22 and 15.33 ± 4.00 were the mean NIHSS scores at admission and discharge respectively, in patients with poor clinical outcomes (i.e., final mRS >2) [Table 1].
The mean ASPECTS score in patients with anterior circulation acute ischemic stroke was 8.13 ± 1.74 (Range: 3-10). The mean ASPECTS scores in patients with good and poor clinical outcomes were 9.00 ± 0.71 & 6.11 ± 1.76, respectively. Its ability to predict clinical outcomes was statistically significant, with a p-value of <0.001 by the Wilcoxon–Mann–Whitney U test [Tables 1 and 2].
| Predictor | AUROC | 95% CI | P | Sn | Sp | PPV | NPV | DA |
|---|---|---|---|---|---|---|---|---|
| ASPECT Score | 0.971 | 0.925-1 | <0.001 | 86% | 100% | 100% | 75% | 90% |
| Tann’s score on sCT | 0.754 | 0.564-0.943 | 0.010 | 81% | 67% | 85% | 60% | 77% |
| Menon’s score on sCT | 0.810 | 0.636-0.983 | 0.002 | 81% | 78% | 90% | 64% | 80% |
| Menon’s score on mCT | 0.815 | 0.643-0.987 | 0.002 | 81% | 78% | 90% | 64% | 80% |
AUROC: Area under ROC curve; CI: Confidence interval; P: P value; Sn: Sensitivity; Sp: Specificity; PPV: Positive predictive value; NPV: Negative predictive value; DA: Diagnostic Accuracy, ASPECT: Alberta stroke program early CT score, sCT: single-phase CT angiography, mCT: multiphase CT angiography.
The area under the ROC curve (AUROC) for Tan score on sCT, Menon score on sCT, and Menon score on mCT in predicting clinical outcome were 0.754 (95% CI: 0.564 - 0.943), 0.81 (95% CI: 0.636 - 0.983) and 0.815 (95% CI: 0.643 - 0.987) with statistically significant p-value as mentioned in Tables 2 and 3.
On follow-up cross-sectional imaging, the collateral status on sCT and mCT showed a negative correlation with final infarct volume. The Spearman correlation coefficient was –0.73, –0.81, and –0.79 for Tan’s score on sCT, Menon’s score on sCT, and Menon’s score on mCT, respectively, in predicting radiological outcomes [Table 3].
| Scoring systems | Clinical outcome | Radiological outcome | ||||
|---|---|---|---|---|---|---|
| Good | Poor | Wilcoxan Man Whitney U test | Spearman correlation coefficient | p-value | ||
| Mean (SD) | Mean (SD) | W | p-value | |||
| Tann’s score on sCT | 2.76 (0.54) | 2.00 (0.87) | 142.500 | 0.010 | –0.73 | <0.001 |
| Menon’s score on sCT | 4.62 (0.86) | 3.22 (1.20) | 153.000 | 0.002 | –0.81 | <0.001 |
| Menon’s score on mCT | 4.67 (0.73) | 3.33 (1.22) | 154,000 | 0,002 | –0.79 | <0.001 |
DISCUSSION
In our prospective observational study, the mean age of anterior circulation acute ischemic stroke presentation was 56.53 ± 14.28 years and 56.25 ± 19.26 years for males and females, respectively.
Though hypertension and diabetes mellitus were more commonly encountered risk factors in our study, no single vascular risk factor, including smoking, diabetes, hypertension, coronary arterial disease, or previous stroke or TIA, was statistically significant in the prediction of collateral status [Table 3] similar to the result of the previous study by Menon et al. in 2011.11 In a study by Adhithyan R. in 2021,12 it was shown that patients of hypertension and hyperglycemia were more prone to have poor collateral status, but was not statistically significant. No significant association of smoking and atrial fibrillation to collateral status was noted in both sCT and mCT other studies.13, 14
Patients with good sCT and mCT collateral scores had good initial clinical mean NIHSS scores (7.5 on Tan scoring, 6.05 on sCT Menon scoring, and 6.68 on mCT Menon). These patients also had good initial radiological status in the form of NCCT ASPECTS score (>8). These findings are in congruence with previous studies.11,12,15,
Collateral status on both sCT and mCT showed a negative correlation with the final infarct volume, which was calculated on follow-up cross-sectional imaging using the voxel-based method. Also, no significant difference in collateral status on sCT and mCT in the outcome prediction was noted. Among sCT collateral scores, the Menon score for sCT performed slightly better than the traditional Tan score.
Results are similar to the study by Schregel et al.,16 which revealed all collateral scores correlated well with each other and CTP parameters. An sCT collateral score discriminated best between favorable and unfavorable outcomes as determined by the modified Rankin Scale three months after stroke.
In a retrospective study by Wang et al.,17 both single and multiphase collateral scores had moderate negative correlation with final infarct volume (r = -0.43, p- 0.001; r = -0.44, p-0.001) and multiphase Menon score predicted slightly better than single phase Menon score [Area under the curve (AUC), 0.72 vs 0.64; P = 0.045]. The Spearman correlation coefficient was –0.23; –0.39 for sCT; mCT in patients who underwent immediate reperfusion vs –0.43; –0.52 for sCT; mCT in patients who did not undergo immediate reperfusion therapy.17
In our study, scoring systems were analyzed with the Receiver operating curve for final mRS with results as mentioned in tables 1 and 2. 95.2% (Tan score) and 85.7% (Menon score) of patients with good collaterals on sCT were shown to have a sound clinical outcome. While 85.7% (Menon score) of patients with good collaterals on mCT were functionally independent. This indicates no significant difference in outcome prediction by collateral status on sCT and mCT.
In a study by Adhithyan R.12, cut-off scoring of 34 in mCT scoring was shown to predict good functional independence with sensitivity and specificity of 82.4% and 83.3%, respectively. Similarly, a cut-off of 32 in sCT was shown to predict good clinical outcomes with sensitivity and specificity of <50%
The cut-off of collateral scoring methods in the prediction of good functional outcomes is not described in any other studies. Thus, further work in this direction (i.e., including more patients with diverse collateral status) is needed to standardize the cut-off of greater accuracy. Also, because many other factors play a role in clinical outcomes (like medical intervention, comorbidities, etc.), absolute cut-off might not be possible to determine or may not be applicable to clinical scenarios.
None of the patients with good collateral status revealed hemorrhagic transformation. Few studies in the past have also shown that good collateral status is less vulnerable to undergo the symptomatic intracranial hemorrhage (sICH).18,19,20
Only one patient in our study with poor collateral status had symptomatic intra-cerebral hemorrhagic transformation (sICH) of infarct post-endovascular thrombolytic therapy. As we had only one patient with sICH, a statistically significant association of poor collateral status with sICH could not be established.
As multiple factors play a role in the prediction of the patient’s clinical outcome, multivariate correlation with final clinical outcome revealed, in addition to collateral status, ASPECT score on NCCT and NIHSS at admission showed statistically significant p-value (<0.001). Age and gender did not show any significant correlation in outcome prediction, as depicted in Table 1.
In a previous study by Wang et al.,17 age showed a statistically significant correlation in outcome prediction, with the mean age of patients being higher in those with poor clinical outcomes than with good clinical outcomes. In our study, the mean age of patients with poor clinical outcomes was higher but not statistically significant.
Most of the patients who presented to us were beyond the window period for IV thrombolysis and were conservatively managed. In a recent retrospective study by Ban et al.,13 collateral status was evaluated in cases of late window period (24–72 hrs) middle cerebral artery stenosis using CT perfusion, which revealed the status of collateral blood flow influences the severity of neurological damage and size of ischemic stroke lesions. The AUC for Cerebral blood volume ratio, cerebral blood flow ratio, and Tan score were 0.922, 0.779, and 0.709, respectively, in predicting good functional outcome (mRS score £2).13
Although studies have shown no significant relation between the time of imaging and collateral status, an improved comprehension of the temporal relationship between collateral status & tissue outcome will necessitate serial imaging over time, including at time points later than 6 hours.11
Our study has a few limitations. The sample size was small. None of our patients could undergo a thrombectomy procedure. Hence, we could not assess this group of patients, which is the central focus of collateral status evaluation.
CONCLUSION
Both Multiphase CTA and Conventional Single phase arterial CTA showed statistically significant results in clinical and radiological outcome prediction. Although most of our patients had not undergone any reperfusion therapy, good collateral status had shown a strong association in predicting good clinical outcomes.
Ethical approval
This observational, prospective study was conducted in a tertiary care hospital after getting necessary approvals from Research Review Committee and Institutional Human Ethics Committee Ref no: 2020/PG/Jan/24 dated 24.11.2020.
Declaration of patient consent
Informed consent was obtained from every patient before their recruitment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
References
- Stroke incidence, mortality, subtypes in rural and urban populations in five geographic areas of India (2018–2019): results from the National Stroke Registry Programme. Lancet Reg Health - Southeast Asia. 2023;100308
- [Google Scholar]
- Stroke in India: A systematic review of the incidence, prevalence, and case fatality. Int J Stroke. 2022;17:132-40.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Ischemic stroke. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. [Last accessed 2022 Mar 7]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK499997/
- [Google Scholar]
- Multimodal CT techniques for cerebrovascular and hemodynamic evaluation of ischemic stroke: occlusion, collaterals, and perfusion. Expert Rev Neurother. 2016;16:515-25.
- [CrossRef] [PubMed] [Google Scholar]
- Collateral Scores in Acute Ischemic Stroke : A retrospective study assessing the suitability of collateral scores as standalone predictors of clinical outcome. Clin Neuroradiol. 2020;30:789-93.
- [CrossRef] [PubMed] [Google Scholar]
- Multiphase CT angiography: A new tool for the imaging triage of patients with acute ischemic stroke. Radiology. 2015;275:510-20.
- [CrossRef] [PubMed] [Google Scholar]
- CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol.. 2009;30:525-31.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol.. 2001;22:1534-42.
- [PubMed] [PubMed Central] [Google Scholar]
- National institutes of health stroke scale (NIHSS) J Physiother.. 2014;60:61.
- [CrossRef] [PubMed] [Google Scholar]
- Outcomes validity and reliability of the modified rankin scale: Implications for stroke clinical trials. Stroke. 2007;38:1091-6.
- [CrossRef] [PubMed] [Google Scholar]
- Regional leptomeningeal score on CT Angiography predicts clinical and imaging outcomes in patients with acute anterior circulation occlusions. Am J Neuroradiol. 2011;32:1640-5.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Assessment of collaterals using multiphasic CT angiography in acute stroke: Its correlation with clinical outcomes. Neurol India.. 2021;69:1586-91.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of collateral status and outcome in patients with middle cerebral artery stenosis in late time window by CT perfusion imaging. Front Neurol.. 2022;13:991023.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- MR CLEAN Investigators. MR CLEAN, a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands: study protocol for a randomized controlled trial. Trials.. 2014;15:343.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Single-phase computed tomography angiography sufficiently predicts outcomes after mechanical thrombectomy. J Chin Med Assoc. 2020;83:478-83.
- [CrossRef] [PubMed] [Google Scholar]
- Outcome prediction using perfusion parameters and collateral scores of multi-phase and single-phase CT angiography in acute stroke: Need for one, two, three, or thirty scans? J Stroke. 2018. ;20:362-72.
- [Google Scholar]
- Collateral status at single-phase and multiphase CT Angiography versus CT perfusion for outcome prediction in anterior circulation acute ischemic stroke. Radiology. 2020;296:393-400.
- [CrossRef] [PubMed] [Google Scholar]
- MR CLEAN investigators. Collateral status on baseline computed tomographic angiography and intra-arterial treatment effect in patients with proximal anterior circulation stroke. Stroke.. 2016;47:768-76.
- [CrossRef] [PubMed] [Google Scholar]
- Predictors of hemorrhage following intra-arterial thrombolysis for acute ischemic stroke: The role of pial collateral formation. AJNR Am J Neuroradiol.. 2009;30:165-70.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- UCLA-Samsung stroke collaborators. Collateral flow averts hemorrhagic transformation after endovascular therapy for acute ischemic stroke. Stroke.. 2011;42:2235-9.
- [CrossRef] [PubMed] [Google Scholar]







