Translate this page into:
Clinico-pathological Study of Breast Lesions with regard to E-Cadherin Immunoexpression as a Biomarker in Breast Cancer Tumorigenesis and Disease Progression
Corresponding Author Dr Kafil Akhtar Professor Department of Pathology, Jawaharlal Nehru Medical College, Aligarh Muslim University (AMU), Aligarh, Uttar Pradesh, India -202002 drkafilakhtar@gmail.com
-
Received: ,
Accepted: ,
How to cite: Nasrin T, Vasenwala SM, Mehdi G, Akhtar K, Anees A. Clinico Clinico-pathological Study of Breast Lesions with regard to E- Cadherin Immunoexpression as a biomarker in Breast Cancer Tumorige-nesis and Disease Progression. Future Health 2023; 1(1):21-27.
Abstract
Introduction-
Proper histopathologic categorization of breast carcinomas has prognostic implications. Recent molecular profiling analysis of lobular compared with ductal cancers show E-cadherin mutation and loss to be a defining feature of lobular breast cancers, and suggest it to be a distinct molecular subtype of breast cancers. The majority of lobular carcinoma in situ and invasive lobular carcinomas have shown a complete loss of E-cadherin expression. E-cadherin loss explains the histopathologic appearance of lobular carcinoma in situ including a diffuse growth pattern of this non-gland-forming tumor with discohesive tumor cells.
Material & Methods-
The present study was conducted on 234 patients presenting with signs and symptoms of breast lesions. A detailed history and complete physical examination and preliminary investigations were recorded, followed by surgical intervention. Histopathology was done on formalin fixed and paraffin embedded tissue sections of breast lesions and subsequently immunostaining with ER, PR, HER2/ Neu and E-cadherin was performed.
Results-
Average age of patient's presentation in the study was 38.1±16.9 years. Two hundred and twenty one cases (94.4%) of breast lesions were seen in females and 13 cases (5.6%) in males, with Male: Female (M:F) ratio of 1:16. Out of the total 234 cases, 108 (46%) were diagnosed as benign and 126 (53.84%) cases as malignant. Out of the total of 126 malignant breast lesions, infiltrating ductal carcinoma (not otherwise specified) was the most frequently diagnosed lesion, comprising 67(53.2%) cases. Most of the cases, 120(95.0%) had a tumor mass. All the benign lesions showed strong and homogenous membranous positivity of E-cadherin with intensity score of 4+. Most of the Grade 1 infiltrating duct carcinomas showed E-cadherin 3+ proportion score with gradually decreasing score in higher grades. All the 6 cases (100%) of infiltrating lobular carcinoma were negative for E-cadherin. Infiltrating duct carcinomas with medullary features showed 1 case each of weak 1+ proportion score and 2+ Intensity score in < 10% of cells. E-cadherin scoring in benign and malignant cases was statistically significant (p value = 0.0169).
Conclusions-
E-cadherin expression can be used as diagnostic modality for the sub-typing breast cancers as Infiltrating duct or lobular carcinomas, if morphology is not clear, with a definite prognostic significance.
Keywords
Breast
Cancer
E-cadherin
Immunoexpression
lymphnode
Metastasis
Prognosis
Introduction
The process of cancer invasion and metastasis consists of a complex series of sequential steps, involving specific tumor cells and host properties.1 Detachment of tumor cells from the primary lesion is assumed to be the initial and important step in the metastatic process.2
Vijaya et al described that tumor cells are more easily separated from a solid tumor mass than their counterpart normal cells from surrounding tissue.[2] Their detachment is regulated by the property of tumor cell "adhesiveness." However, the molecular basis of the mutual adhesiveness of cancer cells has not been clarified in vivo, and it is difficult to estimate the actual strength of intercellular connection from the expression of a single adhesive molecule.2
Recently the existence of abnormal E-cadherin expression in human cancerous tissues was demonstrated and a significant relationship was found between E-cadherin expression and histological grade or invasiveness in gastric cancer.3 Proper histopathologic categorization of breast carcinomas has prognostic implications.4 The majority of invasive lobular carcinomas (ILC) have shown a complete loss of E-cadherin expression. The loss of E-cadherin is from the outset, i.e., in the pre-invasive stage of lobular carcinoma in situ (LCIS). E-cadherin loss explains the histopathologic appearance of LCIS including a diffuse growth pattern of this non-gland-forming tumor with discohesive tumor cells.5
Keeping in mind the prognostic factors in breast cancer, this study was carried out to diagnose and grade cases of breast cancer and to evaluate their relationship to E- cadherin expression.
Material & Methods
The present study was conducted on 234 patients presenting with signs and symptoms of breast lesions attending the Out-patient and In-patient clinics of the Department of General Surgery and Department of Pathology. A detailed history and complete physical examination and preliminary investigations were recorded in each case. Consent was taken for surgical intervention by the surgeon incharge. Histopathology was done on formalin fixed and paraffin embedded tissue sections of breast lesions and immunostain for ER, PR, HER2/ Neu and E-cadherin were employed on 20 benign and 60 malignant cases. Positive control taken was normal ductal epithelial cells and negative control as lining of vessels.
Microscopic evaluation was done using Olympus CH21 i-microscope with low (4X and 10X) and a high power field (40X) diameter of 0.44 mm and the corresponding field area of 0.152 mm2. H&E stained histological sections were assigned diagnosis and typed according to WHO classification of tumours of the breast and grading of malignant cases was done as per Modified Scarff-Bloom-Richardson grading scheme.
Results of IHC: E-cadherin showed membranous positivity. E-cadherin scoring was done as done according to the 4-point scale as follows:5
Scoring of Intensity: 0 = Negative; 1 + = Weak and heterogeneous; 2+= Mild or weak and homogeneous; 3+ = Moderate or strong and heterogeneous; 4+ = Intense or strong and homogeneous. Result was interpretated as - Negative score = 0, 1+, 2+ and Positive score = 3+, 4+.
-
Scoring of proportion was calculated on positive percentage of cells, showing membrane expression from 0 - 3 as follows:
Score 0 = complete absence or negative expression; Score 1+ = < 10%; Score 2+ = 10 - 50%; Score 3+ = > 50%. Result was interpretated as Negative score = 0 and Positive score = 1+, 2+, 3+.
Statistical analysis tests employed was Fischer exact test showed that the probability of obtaining any such set of values was given by the hypergeometric distribution:
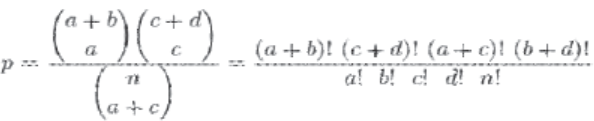
In this study, a p value of < 0.01 was considered highly significant, p value between 0.01 to 0.05 was significant and a p value of > 0.05 was considered insignificant.
Results
The average age of presentation of patients was 38.1±16.9 years. Out of the total 234 cases, 108 (46%) were diagnosed as benign and 126 (53.84%) cases as malignant. Majority of the benign breast lesions, 74 (68.6%) cases occurred before the 4th decade with average age being 27.8±16.9 years. The highest incidence of malignant cases were seen in the age group of 41-50 years (41.9%) with average age of presentation of malignant lesions as 45.96±16.8 years.
A total of 221 cases (94.4%) of breast lesions were seen in females and 13 cases (5.6%) in males with Male: Female ratio was 1:16. Lump or mass in the breast was the commonest complaint, in 218 cases (93.5%) followed by skin ulceration in 30 cases (12.8%) and nipple retraction was seen in 16 cases (6.8%).
Out of the total 234 cases, 108 (46%) were diagnosed as benign and 126 (53.84%) cases as malignant. Out of a total of 108 benign breast cases, fibroadenoma was the most frequently diagnosed subtype comprising 60 cases (55.6%). This was followed by gynaecomastia in male patients with 12 cases (11.2%); fibrocystic disease and chronic mastitis both 8 (7.4%) cases each; 5 (4.6%) cases of breast abscess; duct ectasia 3 (2.7%) cases and fibroadenomatoid hyperplasia 2 (1.8%) cases. Other histopathological types comprised of 2 cases (1.8%) each of atypical ductal hyperplasia, tubercular mastitis and tubular adenoma and 1 case (0.9%) each of benign phyllodes, rhabdomyoma, hydatid cyst and sebaceous cyst.
Out of the total of 126 malignant breast lesions, infiltrating ductal carcinoma (not otherwise specified) was the most frequently diagnosed lesion comprising 67 cases (53.2%). This was followed by residual/ recurrent ductal carcinoma 31 cases (24.6%); 7 cases (5.6%) of malignant phyllodes and 6 cases (4.8%) of infiltrating lobular carcinoma. Other histopathological types consisted of 4 cases (3.2 %) of Paget's disease with IDC, 3 cases of tubular carcinoma (2.4%), 2 cases (1.6%) each of IDC with medullary features and mixed ductal and lobular carcinoma and 1 case (0.8%) each of papillary carcinoma, intracystic papillary carcinoma, invasive micropapillary carcinoma and metaplastic carcinoma.
In most of the malignant cases, tumor size ranged from 2-5cms in 58 cases (46.0%); followed closely by > 5 cms in 51 cases (40.5%). Histological grading was done according to Nottingham modification of the Bloom-Richardson system (1982). Majority of the cases, 53 (42.0%) were of Grade 2, followed by 47 cases (37.4%) of grade 1 and 26 cases (20.6%) of Grade 3.
Expression of E-cadherin was studied in 20 cases of benign morphology. All of them showed strong and homogenous membranous positivity with intensity score of 4+. Taking into account the proportion of positive cells on E-cadherin staining, majority of fibroadenoma cases, 8 (40.0%) showed proportion score 3+, while 2 (10.0%) cases showed 2+. One case of benign phyllodes showed 2+ positivity and intensity score of 2+. Majority of gynaecomastia cases, 4 (20.0%) showed 3+ proportion score while 1 case showed 2+ score . One case each of chronic mastitis and fibrocystic disease showed of 3+ and 2+ proportion score.
Infiltrating ductal carcinoma showed significant association between grade and expression of E- cadherin. Most of the Grade 1 IDC showed 3+ proportion score, with gradually decreasing score in higher grades. Out of 18 cases of Grade 1 IDC NOS, 12 (66.6%) showed 3+ proportion score (Figure 1), 5 (27.8%) cases showed 2+ score whereas 1 (5.6%) case showed 1+ score (Table 1). Out of 15 cases of Grade 2 IDC NOS, 7 (46.7%) showed 3+ and 6 (40%) showed 2+ proportion score with 2 cases (13.3%) showing 1+ score. Out of 10 cases of Grade 3 IDC NOS, 7 (70%) cases showed 2+ proportion score and 3 cases (30%) showed 1+, none showed 3+ score (Table 1). On applying Fisher Exact probability test between grade and E-cadherin score, p value was 0.0059 which was statistically significant (Table 1).
| Type of lesion | No of cases | Degree of E-cadherin expression | ||||
|---|---|---|---|---|---|---|
| 0 (completely negative) | 1 + (<10% cells) | 2+ (10- 50%cells) | 3+ (>50%cells) | |||
| Infiltrating Ductal Carcinoma (IDC)- Grade 1(Well Differentiated) | 18 | - | 01 | 05 | 12 | P = .0059 |
| IDC Grade 2 (Moderately differentiated) | 15 | - | 02 | 06 | 07 | |
| IDC Grade 3 (Poorly differentiated) | 10 | - | 03 | 07 | - | |
| Invasive lobular carcinoma | 06 | 06 | - | - | - | |
| IDC with Medullary features | 02 | 01 | 01 | - | - | |
| Mixed ductal and Lobular carcinoma | 02 | 01 | - | 01 | - | |
| Residual/recurrent IDC | 02 | - | - | 01 | 01 | |
| Metaplastic carcinoma | 01 | - | - | 01 | - | |
| Malignant phylloides | 02 | 01 | 01 | - | - | |
| Pagets disease with IDC | 02 | - | - | 01 | 01 | |

- Infiltrating Ductal Carcinoma Grade 3: Showing moderately positive duct cells with complete membranous staining (3+) in 10-50% of cells (2+) (IHC E- cadherin x 40).
An our study, all the 6 cases (100%) of infiltrating Inhularnarninnma were nenafive fnr F-naHherin (Figure 2) (Table 1).

- Infiltrating Lobular Carcinoma: Showing negative immunostaining with E-cadherin (IHC E- cadherin x 40).
IDC with medullary features showed 1 case each of weak 1+ proportion score staining and 2+ Intensity score in <10% of cells. Two cases (100%) of combined ductal and lobular carcinoma of breast showed 2+ proportion score in the ductal portion in both the cases, whereas lobular portion showed complete absence of E-cadherin expression (Table 1). 2 cases (6.4%) out of 31 cases of recurrence of IDC NOS were stained, 1 case showed 2+ proportion score and the other showed 3+ score. 1 case (100%) of metaplastic carcinoma stained showed 2+ proportion score. Two cases of malignant phyllodes were stained and showed positive membranous staining in epithelial lining in both the cases, but the atypical spindle cells showed negative staining (Figure 3).

- Malignantphylloides tumor: Showing crowded spindle cells negative for E-cadherin (IHC E-cadherin x 40).
Two cases of Pagets disease with IDC NOS, showed strong 3+ proportion scoring of tumor cells in skin as well as in the underlying malignant cells.
On applying Fisher Exact test on E-cadherin scoring on benign and malignant cases overall, p value was found to be 0.0169, which was statistically significant. It implies stronger expression of E-cadherin in benign lesions as compared to malignant ones. No association was found between E-cadherin expression and the clinicopathologic features (Table 2).
| VARIABLES | E-cadherin positivity | E-cadherin negativity | p-value |
|---|---|---|---|
| Patient's age <30 years | 07 | 02 | |
| 31-40 years | 13 | 04 | |
| 41-50 years | 22 | 03 | 0.18 |
| >51 years | 08 | 06 | |
| Menopausal status | 06 | 01 | 0.69 |
| Positive family history | 02 | 0 | NA |
| Side of primary - Left | 35 | 07 | |
| Side of primary - Right | 13 | 03 | 0.59 |
| Bilaterality | 02 | 01 | |
| Size of tumor | |||
| <2 cm | 09 | 0 | 0.22 |
| 2-5 cm | 22 | 04 | |
| >5 cm | 19 | 07 | |
| SBR Grade 1 | 23 | 05 | |
| SBR Grade 2 | 15 | 05 | 0.71 |
| SBR Grade 3 | 12 | 02 |
On applying fisher exact test, no association was seen between lymph node status and E-cadherin expression (Table 3). No statistically significant association was found between ER and PR status and E-cadherin
| Variables | No. of cases | E-cadherin positive | E-cadherin negative | p-value |
|---|---|---|---|---|
| Lymph nodes | 42 | 32 | 10 | 0.59 |
| ER + | 22 | 13 | 02 | |
| ER- | 33 | 19 | 03 | 0.68 |
| PR+ | 17 | 11 | 02 | |
| PR- | 38 | 21 | 03 | 0.58 |
| HER-2 + | 20 | 16 | 0 | 0.046 |
Discussion
In the present study the average age at diagnosis of all breast lesions was found to be38.1±16.9 years. This included both benign and malignant cases. Majority of the benign breast lesions 74 (68.6%) cases occurred before 4th decade with average age being 27.8±16.9 years. This can be attributed to high incidence of fibroadenoma at a younger age, as it comprised of 62 (57.4%) benign cases. Majority of the cases of fibroadenoma belonged to 2ndand 3rddecades (16- 30yrs). The reason may be hormonal dependency, participation in lactation and involution at menopause which is a possible contribution to lump formation and evolution in fibroadenoma.1 Furthermore, the mean age of fibroadenoma in teenagers in India is reported as 14 years viz -a -viz 11 years in Germany.1 This implies that occurrence of fibroadenoma is more common in teenagers.
Average age at diagnosis for breast carcinoma was 45.96±16.8 years in our study. According to Sahoo et al, breast cancer has a bimodal age distribution with a dip around menopause followed by a rise after 65years of age.4 But in our study, only 9 (7.3%) cases belonged to age group >60 years. In a study conducted in India by Vijaya et al it was found that incidence appears to decrease with age after the age of 50 years.2 This shift towards younger age of patients has been associated with effect of hormones, delayed pregnancy, increased awareness amongst patients about cancer and prompt availability of medical assistance.
Out of a total of 126 malignant breast lesions, IDC NOS was the most frequently diagnosed lesion, comprising 67 cases (53.2%). Our result was comparable to studies by Lukohetty et al.6 Multiple independent studies have shown that modified Scarff-Bloom- Richardson(SBR) grading system has an independent prognostic value that is equivalent to that of lymph node status and greater than that of tumor size.2,6 Patients with grade III tumors have 4.4 times increased risk of recurrence as compared to those with grade I tumors.4 Taking into account the proportion of positive cells on E-cadherin staining, majority of cases of fibroadenoma, 8 (40.0%) showed proportion score 3+, while 2 (10.0%) cases showed 2+ score. Similarly majority of cases of gynaecomastia, 4 (20.0%) showed 3+ proportion score and a single case of benign phyllodes showed 2+ positivity and intensity score of 2+. Our findings are consisted with the work of Hollestelle et al, who reported strong homogenous E-cadherin positivity in benign lesions of breast.7
We stained 60 cases of carcinoma breast for E- cadherin. Infiltrating ductal carcinoma (IDC NOS) showed significant association between grade and expression of E-cadherin (Table 1). 3+ proportion score was seen mostly in grade 1 cases, with gradually decreasing score in higher grades. Out of 18 cases of Grade 1 IDC NOS, 12 (66.6%) showed 3+ proportion score and out of 15 cases of Grade 2 IDC NOS, 7 (46.7%) showed 3+ and 6 (40.0%) showed 2+ proportion score (Table 1). Out of 10 cases of Grade 3 IDC NOS, 7 (70.0%) cases showed 2+ proportion score. A statistically significant finding (p=0.0059) was seen between grade and E-cadherin score in our study (Table 1). Our findings were consistent with studies of Theys et al and Felipe et al, who reported that reduced expression of E-cadherin was associated with poor differentiation and higher grade of malignancy.8,9
On applying Fisher Exact test on E-cadherin scoring on benign and malignant cases overall, p value was found to be 0.0169, which was statistically significant. It implies stronger expression of E-cadherin in benign lesions as compared to malignant ones. Our findings were consistent with Hollestelle et al.7
On correlating expression of E-cadherin with various clinical parameters, no correlation was seen with patients age (p=0.18), menopausal status (p=0.69) and location of breast lesions (p=0.59) (Table 2). Our findings were in accordance with Kasangian et al and Acs et al. 10,11
On comparing gross findings with E-cadherin expression, again no correlation was seen with size of tumor (p=0.22) and SBR grading including all types of cancer (p=0.71) (Table 2). However significant association was seen between IDC NOS grading and E-cadherin expression (p=0.0059) (Table 1). Our results are comparable to studies done by Loh et al and Kim et al.12,13
No association was found on comparing number of metastatic lymph nodes status and E-cadherin expression. Out of 60 malignant, 42 showed lymph node metasis and when stained for E-cadherin showed positive expression in 32 cases and negative expression in 10 cases (p=0.59) (Table 3). Contrary to our findings, tumors with low level of E-cadherin show lymph node metastasis.12,13 This can be due to the hypothesis that E-cadherin expression could be unstable in some tumors and expression may locally or temporarily diminish, leading to metastasis and later reactivation may cause E-cadherin positivity.14 Another possibility is that tumor cells express E-cadherin but do not function normally.13
We also correlated the E-cadherin expression with ER, PR and HER-2/neu expression. In our study, no association was seen between ER, PR status and E- cadherin expression (p=0.68 and 0.58 respectively) (Table 3). Various studies have observed positive correlation between reduced E-cadherin expression and ER, PR status.11,13 However others have found no association between ER, PR status and E-cadherin expression.9,10
In our study, the only immunohistochemical marker that showed statistically significant association with E- cadherin expression was HER-2 neu (p=0.046) (Table 3). Our findings were comparable to that of Wang et al and Nora et al.15,16 It could be attributed to the hypothesis that oncogenic pathway mediated by c- erbB-2/HER-2/neu may affect the E-cadherin expression in most invasive ductal breast carcinomas in vivo.17
Conclusions
The immunoexpression of E-cadherin is associated with benign or malignant nature of breast lesions, with expression stronger in benign tumors. It can be used as diagnostic modality for the sub-typing breast cancers as infiltrating duct or lobular carcinoma, if morphology is not clear. Aberrant E-Cadherin expression has been linked to invasiveness and poor prognosis of breast cancers. Our findings support the notion that E- Cadherin loss promotes the development of metastasis and invasiveness. It has a prognostic value in infiltrating duct carcinoma grading and may have an association with Her-2/neu expression which may aid in guiding and monitoring effective treatment.
References
- Progress in the clinical detection of heterogeneity in breast cancer. Cancer Med. 2016;5(12):3475-3488.
- [CrossRef] [PubMed] [Google Scholar]
- A Clinico- pathological study of carcinoma of breast. Int J Surg. 2020;4(2):344-348.
- [CrossRef] [Google Scholar]
- Gastric cancer epidemiology and risk factors. Clin Surg Oncol. 2016;59(4):651-672.
- [CrossRef] [PubMed] [Google Scholar]
- Clinico-Pathological study of carcinoma breast in a tertiary care hospital from West Bengal, India: A Cross-Sectional Observational study. Asian J Med Sci. 2021;12(9):75-78.
- [CrossRef] [Google Scholar]
- E-cadherin expression in molecular types of breast carcinoma. Rom J Morphol Embryol. 2013;54(2):267-73.
- [Google Scholar]
- World Health Organisation Classification of Tumors. Breast Tumours. International Agency for Research on Cancer (IARC) Press, Lyon, France 2019:8-10.
- [Google Scholar]
- Loss of E- cadherin is not a necessity for epithelial to mesenchymal transition in human breast lesions. Breast Canc Res Treat. 2013;138(1):47-57.
- [CrossRef] [PubMed] [Google Scholar]
- E-Cadherin loss associated with EMT promotes radioresistance in human tumor cells. Radio Oncol. 2011;99(3):392-97.
- [CrossRef] [PubMed] [Google Scholar]
- The prognostic role of tumor size in early breast cancer in the era of molecular biology. PloS One. 2017;12(12):189-197.
- [CrossRef] [PubMed] [Google Scholar]
- Differential expression of E-cadherin in lobular and ductal neoplasms of the breast and its biologic and diagnostic implications. Am J Clin Pathol. 2018;115:85-98.
- [CrossRef] [PubMed] [Google Scholar]
- The E-cadherin and N- cadherin switch in epithelial-to-mesenchymal transition: signaling, therapeutic implications, and challenges. Cells. 2019;8(10):1118.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics and prognosis of 17 special histologic subtypes of invasive breast cancers according to World Health Organization classification: comparative analysis to invasive carcinoma of no special type. Breast Canc Res Treat. 2020;184(2):527-542.
- [CrossRef] [PubMed] [Google Scholar]
- The prognostic role of tumor size in early breast cancer in the era of molecular biology. PloS one. 2017;12(12):e0189127.
- [CrossRef] [PubMed] [Google Scholar]
- Induced tamoxifen resistance is mediated by increased methylation of E- cadherin in estrogen receptor-expressing breast cancer cells. Scientific Reports. 2019;9(1):1-7.
- [CrossRef] [PubMed] [Google Scholar]
- The role of E-Cadherin expression in primary site of breast cancer. Arch Gynecol Obstetr. 2022;305:913-920.
- [CrossRef] [PubMed] [Google Scholar]
- Epithelial paradox: clinical significance of coexpression of E-cadherin and vimentin with regard to invasion and metastasis of breast cancer. Clin Breast Cancer. 2018;18(5):1003-1009.
- [CrossRef] [PubMed] [Google Scholar]







