Translate this page into:
Role of MRI in diagnosis of atypical Parkinsonian syndrome: A case series with brief review of literature
* Corresponding author: Yada Varun Tej, Department of Radiodiagnosis, AIIMS, Bhopal, India yadavarunteja2@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Tej YV, Surana H, Ahuja M, Bhagat AC, Sharma J. Role of MRI in diagnosis of atypical Parkinsonian syndrome: A case series with brief review of literature. Future Health. 2024;2:65–68. doi: 10.25259/FH_5_2024
Abstract
We describe three cases of patients with atypical Parkinsonian syndrome (APS), who presented with gait disturbance, postural instability, decreasing facial expression, dyskinesia, and subjective cognitive impairment. The patients underwent magnetic resonance imaging (MRI) Brain for differential diagnosis of APS. MRI made it possible to offer a suggestive diagnosis and determine individual strategic management for patients with APS.
Keywords
Atypical Parkinsonian syndrome
Progressive supranuclear palsy
Multiple system atrophy
MRPI
Parkinsonism
INTRODUCTION
Parkinsonism is a neurological syndrome that manifests with symptoms such as resting tremors, bradykinesia, rigidity, and postural instability. Atypical Parkinsonian syndrome (APS) is a heterogeneous group of neurodegenerative diseases such as progressive supranuclear palsy (PSP), multiple system atrophy (MSA), cortico-basal degeneration (CBD) and dementia with Lewy bodies (DLB). In all of them, the core symptoms of Parkinsonism are accompanied by additional symptoms atypical for idiopathic Parkinson’s disease (PD).1 Due to overlapping features of the APS spectrum, there is a requirement for additional imaging tests to aid in diagnosis. Routine MRI is a method recommended to exclude symptomatic causes of parkinsonism and to differentiate PD from APS.
CASE REPORTS
Case-1: A 54-year-old female came with complaints of dementia, bladder dysfunction, abnormal eye movements, and frequent falls for two years. On examination, slow and hesitant speech was noted with mild slurring. Vertical gaze restriction was present, and the gag reflex was absent. Autonomic function test showed abnormal cold pressor test. With the clinical suspicion of APS, an MRI brain was requested. On mid-sagittal images [Figure 1], severe disproportionate atrophy of the midbrain giving hummingbird sign was noted with MRPI (Magnetic Resonance Parkinsonism Index)- 14.5 (cutoff limit of 13.55) and MRPI 2.0-2.8 (Cutoff limit of 2.18).2 Rounded margins of cerebral peduncles and concave lateral margins of the tegmentum were seen on axial T2W and fluid attenuated inversion recovery (FLAIR) images. Based on the clinical and radiological imaging findings, a diagnosis of Progressive supranuclear palsy (PSP) was made.
- Showing Disproportionate atrophy of the midbrain tegmentum with the enlargement of the third ventricle in a case of Progressive supranuclear palsy, 1a: Axial T2WI, 1b: Mid sagittal T1WI, 1c: Axial FLAIR, 1d: Midsagittal T2WI. Images 1a and 1c show lateral concavity of the tegmentum representing the morning glory sign. Images 1b and 1d show Midbrain atrophy with the superior concavity of the midbrain representing the hummingbird sign.
Case-2: A 68-year-old female came with complaints of dysarthria for five months and was unable to stand (gait ataxia). There was also a history of involuntary movements in bilateral upper and lower limbs for one year and slowness of movements for two years. Bowel and bladder functions were normal. The patient did not have any sensory complaints. The finger nose test was impaired with increased bilateral upper limb tone. With the clinical suspicion of APS, the patient was sent for an MRI brain. On imaging [Figures 2a and 2b], cruciform T2/FLAIR hyperintensity was noted at the level of the pons in the axial plane, giving a “hot cross bun sign”, with disproportionate atrophy of pons and cerebellum compared to the midbrain. In addition, there was significant atrophy of the middle cerebellar peduncle with subtle T2/FLAIR hyperintensity. However, the parkinsonism indices to rule out progressive supranuclear palsy were within normal range. Clinical features and radiological imaging findings were in keeping with the diagnosis of Multiple system atrophy-cerebellar variant (MSA-C).
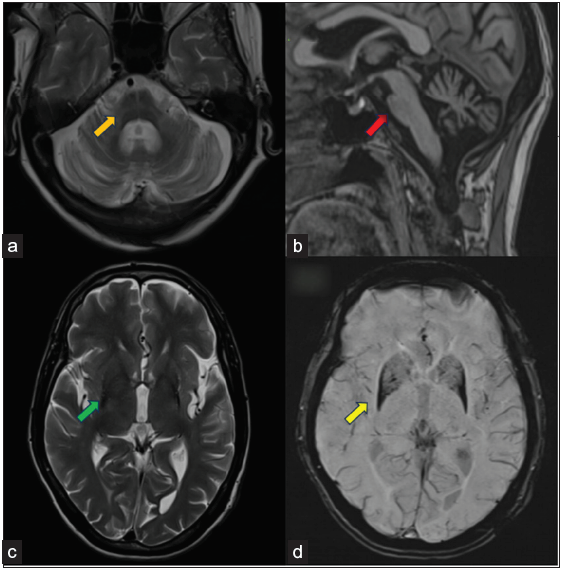
- Showing Multiple system atrophy (a: Axial T2W at pons level, b: Mid Sagittal T1WI, c: Axial T2WI at ventricle level, d: Axial SWI). Images a and b show findings of Multiple system atrophy-cerebellum type (MSA-C) in the form of cruciform T2 hyperintensity in pons described as a hot cross bun sign (orange arrow in image a) and disproportionate pons, cerebellum, and middle cerebellar peduncle atrophy with relatively preserved midbrain volume (red arrow in image b). Images c and d show findings of MSA-P in the form of bilateral putaminal atrophy, hypointense signals on T2WI (green arrow in image c), and SWI blooming (yellow arrows in image d). There were also subtle rim T2 hyperintense signals in putaminal regions (better appreciated on the left side).
Case-3: A 62-year-old male patient came with complaints of bradykinesia, rigidity, and gait difficulty for two years with recent falls. No sensory or autonomic involvement was noted. On MRI brain [Figures 2c and 2d], there was bilateral asymmetrical (L>R) atrophy of putamina with relative sparing of globus pallidus. The dark putaminal signal on T2 and SWI with the “putaminal rim sign” is appreciated on the lateral margin of the left putamen. Based on these findings, a possible diagnosis of Multiple system atrophy- parkinsonian variant (MSA-P) was made.
DISCUSSION
APS is a heterogeneous group of neurodegenerative disorders that comprises MSA, PSP, CBD, and DLB. It has a wide range of symptoms, such as cognitive disorders, aphasia, apraxia, sensory deficits, dysarthria, dysphagia, sphincter disorders, pyramidal syndrome, cerebellar syndrome, or oculomotor disorders, and poor response to levodopa therapy.1,3 Accurate diagnosis of APS is crucial for therapeutic and prognostic significance. Routine 1.5 T MRI of Parkinson’s patients shows no abnormalities in most of the cases. The only characteristic imaging finding of PD is the loss of dorsal hyperintensity within the substantia nigra on T2W images, otherwise known as the absent swallow tail sign.4 Nonetheless, there exist distinct radiological features that aid in distinguishing PD from PSP, MSA, and CBD patients.
The MR sequences to be included for assessing APS are 3D T1W (for regional brain atrophy), susceptibility weighted imaging (SWI) (for iron load), T2, FLAIR (for signal changes), and diffusion weighted imaging (DWI).
Progressive supranuclear palsy is a tauopathy and is the most common form of atypical parkinsonism, with Richardson’s syndrome (PSP-RS) being the most common form of clinical presentation. The key symptoms in PSP-RS are vertical supranuclear gaze palsy, postural instability, and falls. Many other less common clinical phenotypes like PSP with predominant Parkinsonism (PSP-P), progressive gait freezing (PSP-PGF), postural instability (PSP-PI), and frontal presentation (PSP-F), etc. are also seen.5 However, imaging patterns of PSP variants are not clearly understood as most studies focused on PSP-RS only.
Neuronal degeneration in PSP occurs in the midbrain, superior cerebellar peduncle, subthalamus, dentate nucleus, and frontal lobes. Midbrain atrophy with enlargement of the third ventricle is considered a neuropathological hallmark of PSP. On the midsagittal T1 weighted images, there is atrophy of the midbrain with flattening or concavity of the superior margin of the midbrain and preservation of the pons resembling a hummingbird- known as the ‘‘hummingbird sign’’. On axial images, there is concavity of lateral margins of the midbrain tegmentum known as the “morning glory sign”.6
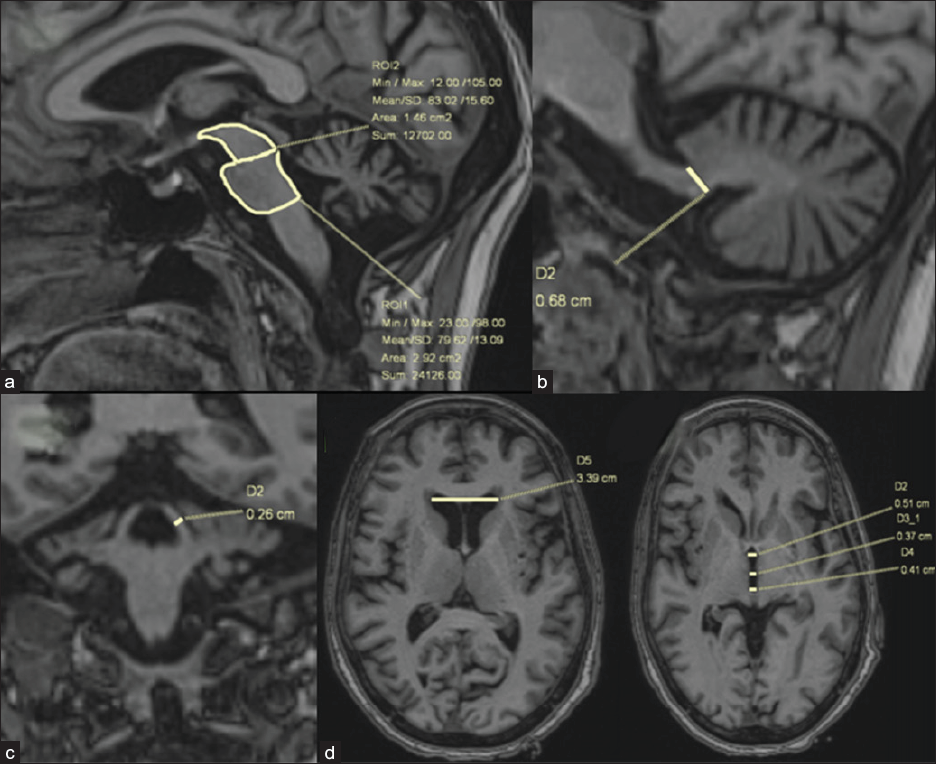
Several manual morphometric indices have been proposed to differentiate PSP from other APS. Out of many such indices, the MRPI [Figure 3] showed better performance for the differentiation of PSP-RS from PD (when > 13.6) and from MSA-P (when > 12.9), with excellent sensitivity and specificity.7 As both superior cerebellar peduncle (SCP) and Midbrain (M) are diminished in PSP patients, the pons/midbrain and MCP/SCP ratios are greater in PSP patients compared to PD or other APS. MRPI 2.0 was recently introduced to improve the discrimination of PSP-P from PD, better than MRPI.2 The MRPI and MRPI 2.0 can be good indicators for monitoring disease progression and can also help distinguish patients in the early stages of PSP, where the clinical picture is misleading.
![(a) MRPI and MRPI 2.0- Three-dimensional T1-weighted images. MRPI combines measurements of the midsagittal surfaces (yellow arrows) of the midbrain and pons and widths of the superior and middle cerebellar peduncles [MRPI = (pons/midbrain) × (middle cerebellar peduncle/superior cerebellar peduncle)]. (b) The limit between the midbrain and pons is defined by the line passing through the inferior border of the inferior colliculus and the ponto-mesencephalic sulcus. The inferior border of the pons is parallel to the previous one. The widths of the superior and middle cerebellar peduncles are measured in the parasagittal (red arrow) and (c) coronal planes (yellow arrows) that best show the peduncles, respectively. The MRPI 2.0 is the product of the MRPI by the ratio of the width of the third ventricle to the largest (d) left-to-right width of the frontal horns of the lateral ventricles (green arrow) on axial views.](/content/174/2024/2/1/img/FH_5_2024-g3.png)
- (a) MRPI and MRPI 2.0- Three-dimensional T1-weighted images. MRPI combines measurements of the midsagittal surfaces (yellow arrows) of the midbrain and pons and widths of the superior and middle cerebellar peduncles [MRPI = (pons/midbrain) × (middle cerebellar peduncle/superior cerebellar peduncle)]. (b) The limit between the midbrain and pons is defined by the line passing through the inferior border of the inferior colliculus and the ponto-mesencephalic sulcus. The inferior border of the pons is parallel to the previous one. The widths of the superior and middle cerebellar peduncles are measured in the parasagittal (red arrow) and (c) coronal planes (yellow arrows) that best show the peduncles, respectively. The MRPI 2.0 is the product of the MRPI by the ratio of the width of the third ventricle to the largest (d) left-to-right width of the frontal horns of the lateral ventricles (green arrow) on axial views.
Multiple system atrophy (MSA) is a synucleinopathy, typically with autonomic dysfunction and variable degree of parkinsonian and cerebellar features. It is broadly subdivided into two main groups- Parkinsonian MSA (MSA-P) and cerebellar MSA (MSA-C). In MSA-P, nigrostriatal degeneration is the predominant pathology, reflected on MR imaging as putaminal body atrophy and T1W/T2W/SWI hypointensity (iron deposition) with rim hyperintensity on T2W images. In MSA-C8, infratentorial structures, and involvement, i.e., olivopontocerebellar atrophy, are more specific. On MR imaging, atrophied pons show areas of increased T2/FLAIR signals affecting transverse pontine fibers, giving the shape of a cross known as a “hot cross bun sign.”9 Other characteristic changes are atrophy and signal alterations of the MCP and cerebellum with dilatation of the 4th ventricle. Although typical imaging findings are described for MSA-P and MSA-C subtypes, considerable overlap occurs.10
CONCLUSION
Neuroimaging for atypical parkinsonism has progressed significantly in recent years, but there is also increasing data on mixed pathologies and overlapping clinical presentations of these disorders. Special MRI sequences and protocols are needed to identify typical findings in APD. Although effective treatments are lacking, biomarkers predicting clinical diagnosis may be valuable if disease-modifying therapies become available.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
Patient consent is not required as the patient’s identity is not disclosed or compromised.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
References
- Diagnostic approach to atypical parkinsonian syndromes. Continuum (Minneap Minn). 2016;22:1117-42.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- A new MR imaging index for differentiation of progressive supranuclear palsy-parkinsonism from Parkinson’s disease. Parkinsonism & Related Disorders. 2018;54:3-8.
- [PubMed] [Google Scholar]
- Chapter three - structural imaging in atypical parkinsonism. In: Politis M, ed. International Review of Neurobiology. Academic Press; 2018. p. :67-148. (Imaging in movement disorders: imaging in atypical parkinsonism and familial movement disorders; vol. 142). Available from: https://www.sciencedirect.com/science/article/pii/S0074774218300771 [cited 2023 Dec 31]
- [Google Scholar]
- The role of magnetic resonance imaging for the diagnosis of atypical parkinsonism. frontiers in neurology 2020:11. Available from: https://www.frontiersin.org/articles/10.3389/fneur.2020.00665 [cited 2023 Dec 31]
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Progressive supranuclear palsy: A case report and brief review of the literature. Radiology Case Reports. 2024;19:250-3.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- The role of neuroimaging in the diagnosis of the atypical parkinsonian syndromes in clinical practice. Neurol Neurochir Pol. 2015;49:421-31.
- [CrossRef] [PubMed] [Google Scholar]
- MR imaging index for differentiation of progressive supranuclear palsy from Parkinson disease and the Parkinson variant of multiple system atrophy. Radiology. 2008;246:214-21.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical and imaging features of multiple system atrophy: challenges for an early and clinically definitive diagnosis. J Mov Disord. 2018;11:107-20.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Multiple system atrophy-cerebellar: A case report and literature review. Radiology Case Reports. 2023;18(3):1121-6.
- [Google Scholar]







