Translate this page into:
MR Imaging and radiological approach in cases of pediatric hemi-cerebral atrophy: A case series with brief review of literature
Corresponding author: Mukesh Kumar, Department of Radiodiagnosis, AIIIMS, Bhopal, India. Mukesh79655@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kumar M, MR Imaging and radiological approach in cases of paediatric cerebral hemiatrophy: A case series with brief review of literature. Future Health. 2024;2:61–64. doi: 10.25259/FH_15_2024
Abstract
Here, we describe three cases of cerebral hemiatrophy, presenting clinically as seizure, contralateral hemiplegia, and cognitive impairment. These patients underwent Magnetic Resonance (MR) imaging along with detailed clinical history for differential diagnosis of cerebral hemiatrophy. Combining clinical and brain imaging findings streamlines diagnosing cerebral hemiatrophy, potentially reducing unnecessary tests and identifying the cause, like Rasmussen’s encephalitis, which can guide treatment (immunotherapy) and improve outcomes.
Keywords
Cerebral hemiatrophy
Sturge–Weber syndrome
Rasmussen
’
s encephalitis
Dyke-Davidoff Masson syndrome.
INTRODUCTION
Cerebral hemiatrophy is a rare neuroimaging finding caused by diverse factors. It results in hypoplasia or atrophy of one of the cerebral hemispheres and leads to symptoms in the contralateral body parts, such as hemiplegia, refractory seizures, and cognitive impairments. Determining the etiology of cerebral hemiatrophy is crucial for definitive treatment and prognosis. Etiologies are typically classified as congenital (resulting from genetic or in-utero insults) or acquired (due to postnatal cerebrovascular lesions, inflammatory processes, or cranial trauma). Acquired cause results in a reduction in volume within an initially healthy cerebral hemisphere. Clinically, the etiologies of cerebral hemiatrophy can be classified into non-progressive (presumed single insult) and progressive (ongoing insult) etiology. Non-progressive etiologies include Dyke–Davidoff–Masson syndrome (DDMS), ischemic or hemorrhagic stroke, cranial irradiation, and cranial trauma. The progressive etiologies include Sturge–Weber syndrome (SWS), Rasmussen’s encephalitis (RE), Parry–Romberg syndrome, germinoma-associated cerebral hemiatrophy, vascular anomalies, and status epilepticus including the hemiconvulsion-hemiplegia-epilepsy [Table 1].1
| Radiological features | Causes |
|---|---|
| 1. Distribution of atrophy | |
| a. Parieto-occipital | SWS |
| b. Perisylvian fronto-temporal | DDMS |
| c. MCA territory | Arterial ischemic stroke |
| 2. Intracranial calcification | |
| a. Gyriform/subcortical (tram-track predominance) | SWS |
| b. Discrete focal calcification | PRS |
| 3. Leptomeningeal enhancement present |
SWS (almost always present) PRS |
| 4. Ipsilateral white matter signal abnormalities | RE |
| 5. Atrophy of ipsilateral caudate nucleus | RE |
| 6. Mass-like lesion with enhancing solid component | Off midline germinoma |
| 7. Vascular anomalies |
Congenital ICA hypoplasia Moya Moya disease |
| 8. Ipsilateral calvaria thickening, enlargement of PNS, and elevated greater wing of the sphenoid | DDMS |
SWS = Sturge–Weber syndrome; DDMS = Dyke–Davidoff–Masson syndrome; MCA = Middle carotid artery; PRS = Parry–Romberg syndrome; RE = Rasmussen’s encephalitis; ICA = internal carotid artery; PNS = Paranasal sinus
CASE REPORTS
Case-1
A 19-year-old female presented with a complaint of right-sided focal seizure since the age of three years. There was no history of hemiparesis. There was also a history of gross development delay. Her birth history and perinatal period were unremarkable. The patient has been on antiepileptics since then. No previous imaging was done. Magnetic resonance imaging (MRI) revealed generalized atrophy with the widening of sulci in the left cerebral hemisphere, predominantly affecting the perisylvian region and the frontotemporal region lobe [Figure 1]. Features of volume loss were also noted in the form of ex-vacuo dilatation of the left lateral ventricle. Compensatory ipsilateral cortical bone hypertrophy in the form of thickening of the calvarium on the left side, enlargement of the frontal sinus on the left side, and elevation of the greater sphenoid wing and petrous ridge were found. Contralateral right side cerebellar atrophy was also seen in the form of prominence of folial spaces, suggesting cross cerebellar diaschisis. No evidence of any leptomeningeal enhancement was seen on the post-contrast scan. Based on the clinical history of predominant perisylvian and frontotemporal involvement and absence of leptomeningeal enhancement, it was diagnosed as DDMS.
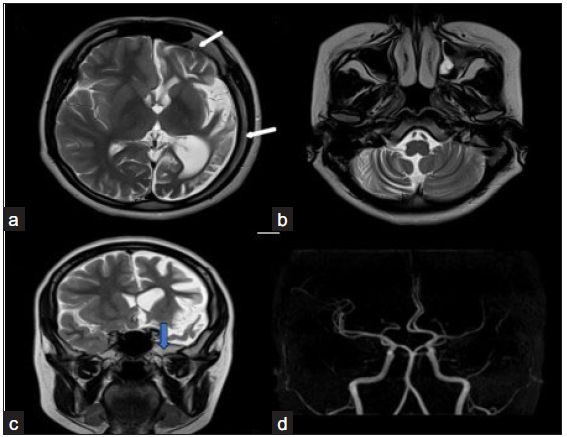
- Dyke–Davidoff–Masson syndrome. (a) T2 axial image shows left cerebral hemiatrophy predominantly involving the perisylvian and temporal region with ex vacuo dilation of the left lateral ventricle. (b) shows hemiatrophy of contralateral cerebellum-crossed cerebellar diaschisis. (c) psilateral calvarial thickening (white arrow) and elevation of the greater wing of the left sphenoid (blue arrow) are seen. Middle cerebral artery Time of Flight (TOF) image (d) shows mild paucity in left Middle cerebral artery (MCA) cortical branches, however internal cerebral artery (ICA) appears normal.
Case- 2
A ten-year-old male child came with a complaint of right-sided focal seizure since the age of five years. There was also a history of episodes of fever and altered sensorium, following which the seizure started. There was no history of associated hemiplegia. Electroencephalogram (EEG) revealed an epileptiform waveform localized to the left side. The birth and developmental histories were normal. MRI revealed diffuse thinning and atrophy of gyri with widening of sulci seen in the left cerebral hemisphere with mild ex-vacuo dilatation of left lateral ventricle suggesting left cerebral hemiatrophy [Figure 2]. There was also mild atrophy of the head of the left caudate nucleus. Atrophy of the left hippocampus with T2/Fluid-attenuated inversion recovery (FLAIR) hyperintense signal was also noted. No compensatory bony hypertrophy was seen. On the post-contrast scan, no leptomeningeal enhancement was seen. Based on clinical and MRI findings, a diagnosis of RE was made.
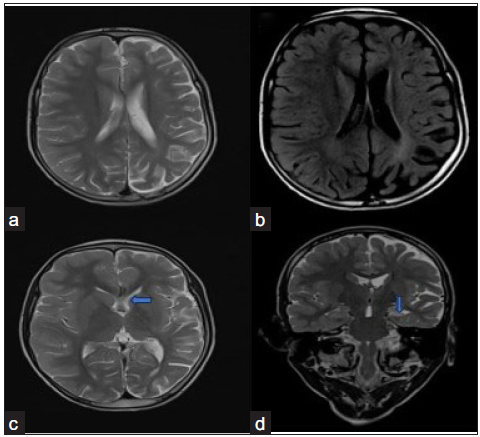
- Rasmussen’s encephalitis. (a) T2 axial (a) image shows mild left cerebral hemiatrophy. (b) There is a mild hyperintense signal seen in the gray and white matter in the fluid-attenuated inversion recovery (FLAIR) axial image. (blue arrow in c) The left caudate nucleus shows mild atrophy. (blue arrow in d) There is also atrophy with altered T2 hyperintense signal seen in the left hippocampus. No contrast enhancement is seen. No compensatory bony calvarial hypertrophy is seen.
Case- 3
An 11-month-old female child presented with a complaint of left-sided tonic-clonic seizure. There was also a history of neuro-regression with loss of motor milestones of sitting without support and neck holding. On examination, a pink salmon stain was seen on the right side of the face (port wine stain). Non-contrast computerized tomography (NCCT) was done outside the hospital, which revealed cerebral atrophy and proper subcortical calcification (images not available). MRI revealed moderate atrophy of the right cerebral hemisphere with disproportionate involvement of the posterior frontal, parietal, occipital, and superior temporal lobes [Figure 3]. It was associated with glio-encephalomalacic changes in the subcortical regions. Post-contrast images showed diffuse pyriform and leptomeningeal enhancement involving almost the entire right cerebral hemisphere. Mild diffuse cerebral atrophy was also seen in the left cerebral hemisphere without any signal changes or abnormal leptomeningeal enhancement. A nonenhancing T1 intermediate and T2 hyperintense subdural collection showing partial suppression on FLAIR is seen over the right frontoparietal convexity, suggesting subdural hemorrhage. Diffuse gyriform blooming is seen in the right cerebral hemisphere in Susceptibility weighted imaging (SWI), which on phase corresponds to calcification. Based on imaging and clinical history, a diagnosis of Sturge–Weber syndrome (SWS) was made.
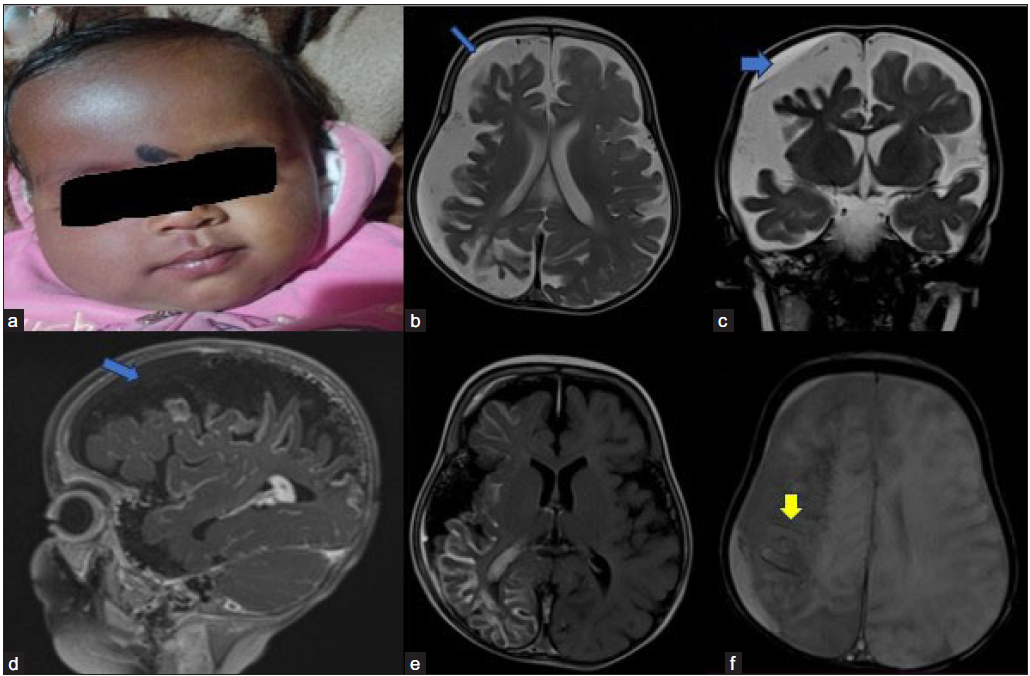
- Sturge–Weber syndrome. Image 1a shows a port wine stain on the right-side face. (3b and 3c blue arrows) Moderate diffuse (with parieto-occipital predominant) right cerebral hemiatrophy seen with mild left cerebral hemiatrophy. (3d blue arrow and 3e) Post-contrast T1 sagittal Fluid-Attenuated Inversion Recovery (FLAIR) axial images show diffuse leptomeningeal enhancement on the right side of the hemisphere. A mild subdural hemorrhage is seen on the right side (blue arrows). (3f) Susceptibility weighted imaging (SWI) image (yellow arrow) shows blooming in the subcortical area on the right side, which is on CT correlation, is suggestive of calcification.
DISCUSSION
The etiologies of cerebral hemiatrophy can be classified into progressive and non-progressive types. The differentiation between nonprogressive etiologies (like DDMS) and progressive etiologies (like SWS and RE) is crucial, as timely treatment of progressive disease will benefit the patient.
DDMS is a rare condition that causes hemiatrophy or underdevelopment of one cerebral hemisphere due to an insult to the developing brain in the fetal or early childhood period. Common symptoms include recurrent seizures, facial asymmetry, contralateral hemiplegia, mental retardation or learning disability, and speech and language disorders. DDMS is usually diagnosed in adolescents and adults, and its radiological features include cerebral hemiatrophy with ipsilateral compensatory hypertrophy of the skull and sinuses, crossed cerebellar hemiatrophy, and no abnormal meningeal enhancement on post-contrast images.2 The presence of calvarial thickening, other bony changes, and predominant perisylvian and frontotemporal patterns of brain involvement suggested the diagnosis of DDMS in the first case.
RE is a rare neurological disorder characterized by focal seizures, hemiparesis, and cognitive decline. It typically occurs in childhood and is associated with anti-glutamate receptor type 3 (GluR3) antibodies. Diagnosis is based on clinical, EEG, and MRI findings. MRI finding includes progressive uni-hemispheric focal cortical atrophy, usually starting in the perisylvian region, T2-weighted/fluid-attenuated inversion recovery (FLAIR) hyperintense signal in the gray or white matter, atrophy of the ipsilateral caudate head, progression of atrophy on serial imaging, and absence of contrast enhancement.3 Unilateral hemispheric atrophy, along with the identification of atrophy of the caudate nucleus head and absence of any associated bony changes, suggested the diagnosis of RE in the second case.
Sturge–Weber Syndrome (SWS) is a congenital neurocutaneous disorder characterized by facial port wine stain (PWS), leptomeningeal angiomatosis, and choroidal angioma. It is caused by mutations in the GNAQ gene, leading to endothelin dysregulation and vascular endothelial dysgenesis. Neuroimaging plays a crucial role in diagnosing SWS, which presents with leptomeningeal enhancement as a cardinal feature on MRI. SWS is associated with various cortical development malformations, with polymicrogyria being the most common.4 Predominant involvement of the parieto-occipital area and the presence of gyriform and leptomeningeal enhancement suggested the diagnosis in the third case.
CONCLUSION
Cerebral hemiatrophy is a rare neuroimaging finding of various etiology, some of which can be progressive. Urgent intervention is recommended when a progressive cause is identified due to potential benefits from timely therapy. Implementing a practical approach that combines clinical and neuroimaging features can streamline the diagnostic process and potentially minimize the need for extensive unnecessary investigations.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCE
- Clinico-radiological approach to cerebral hemiatrophy. Childs Nerv Syst. 2018;34:2377-90.
- [CrossRef] [PubMed] [Google Scholar]
- Dyke-Davidoff-Masson syndrome. J Neurosci Rural Pract. 2012;3:411-3.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Evolution of MRI changes in Rasmussen’s encephalitis. Acta Neurol Scand. 2014;130:253-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sturge-Weber syndrome revisited: the role of neuroradiology. Neuropediatrics. 1996;27:284-94.
- [CrossRef] [PubMed] [Google Scholar]







